| Cas No.: | 158930-17-7 |
| Chemical Name: | Frovatriptan succinate [USAN] |
| Synonyms: | Frovatriptan succinate [USAN];Frova;butanedioic acid,(6R)-6-(methylamino)-6,7,8,9-tetrahydro-5H-carbazole-3-carboxamide,hydrate;Frovatriptan;FROVATRIPTAN SUCCINATE MONOHYDRATE;(+)-(R)-2,3,4,9-Tetrahydro-3-(methylamino)-1H-carbazole-6-carboxamide butanedioate (1:1),monohydrate;(+)-(R)-5,6,7,8-Tetrahydro-6-(methylamino)carbazole-3-carboxamide succinate (1:1),monohydrate;(R)-(+)-6-carbamoyl-N-methyl-2,3,4,9-tetrahydro-1H-carbazol-3-aminium 3-carboxypropanoate monohydrate;1H-Carbazole-6-carboxamide,2,3,;Frovatriptan succinate hydrate;UNII-D28J6W18HY;Frovatriptan succinate;R-(+) 3-Methylamino-6-carboxamido-1,2,3,4-tetrahydrocarbazole monosuccinate monohydrate;Migard;Miguard;Frovelan;(3R)-2,3,4,9-Tetrahydro-3-(methylamino)-1H-carbazole-6-carboxamide Butanedioic Acid Monohydrate;1H-Carbazole-6-carboxamide, 2,3,4,9-tetrahydro-3-(methylamino)-, (R)-, butanedioate (1:1), monohydrate |
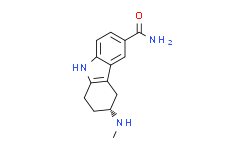
| SMILES: | CN[C@@H]1CCC2=C(C3=CC(C(=O)N)=CC=C3N2)C1 |
| Formula: | C18H25N3O6 |
| M.Wt: | 379.40800 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Frovatriptan succinate hydrate is a potent, high affinity, selective and orally active 5-HT1B, HT1D receptor agonist and a moderately potent 5-HT7 receptor agonist, with pKi values of 8.6, 8.4, and 6.7, respectively. Frovatriptan succinate hydrate is effective in treating the full spectrum of migraine including the associated symptoms of nausea, vomiting, photophobia, and phonophobia. Frovatriptan succinate hydrate can also be used as in mini-prophylaxis in menstrual migraine[1][2]. |
| In Vivo: | Oral bioavailability of Frovatriptan is 22%-30% and is not affected by food. Although the maximum concentration in the plasma is achieved in 2-3 hours, 60%-70% of this is achieved in 1 hour. A steady state is achieved in 4-5 days. Plasma protein binding is low at 15%. The most unique feature is the relative terminal long half-life of about 26 hours. Frovatriptan is chiefly metabolized by CYP1A2 and is cleared by the kidney and liver making moderate failure of either organ not a limiting factor in treatment. Frovatriptan has a low risk of interactions with other drugs[1]. |
| In Vitro: | Frovatriptan succinate hydrate is a potent, high affinity, selective and orally active 5-HT1B, HT1D receptor agonist and a moderately potent 5-HT7 receptor agonist, with pKi values of 8.6, 8.4, and 6.7, respectively. Frovatriptan succinate hydrate is effective in treating the full spectrum of migraine including the associated symptoms of nausea, vomiting, photophobia, and phonophobia. Frovatriptan succinate hydrate can also be used as in mini-prophylaxis in menstrual migraine[1][2]. |
| References: | [1]. Kelman L. Review of frovatriptan in the treatment of migraine. Neuropsychiatr Dis Treat. 2008 Feb;4(1):49-54. [2]. Comer MB. Et al. Pharmacology of the selective 5-HT(1B/1D) agonist frovatriptan. Headache. 2002 Apr;42 Suppl 2:S47-53. |