| Cas No.: | 1332837-31-6 |
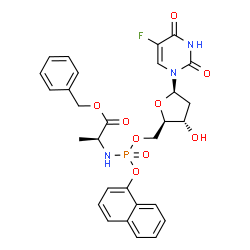
| Chemical Name: | L-Alanine, N-(-2'-deoxy-2',2'-difluoro-p-1-naphthalenyl-5'-cytidylyl)-, phenylmethyl ester |
| Synonyms: | 5-FU analog prodrug NUC-3373, nucleotide analog NUC-3373, phosphoramidate-FUDR-MP prodrug NUC-3373; NUC 3373; NUC3373 |
| SMILES: | C(OCC1=CC=CC=C1)(=O)[C@H](C)NP(C1=C2C(C=CC=C2)=CC=C1)(O)(=O)OC[C@H]1O[C@@H](N2C=CC(N)=NC2=O)[C@H0](F)(F)[C@@H]1O |
| Formula: | C29H30F2N4O8P |
| M.Wt: | 613.52 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | NUC-3373 is a phosphoramidate-based prodrug of the monophosphate form of 5-fluoro-2'-deoxyuridine, the active metabolite of fluorouracil,an antimetabolite fluoropyrimidine analog of the pyrimidine nucleoside, with potential antineoplastic activity. |
| In Vitro: | A phosphoramidate-based prodrug of the monophosphate (MP) form of 5-fluoro-2'-deoxyuridine (FUdR; FUDR), the active metabolite of fluorouracil (5-FU), an antimetabolite fluoropyrimidine analog of the pyrimidine nucleoside, with potential antineoplastic activity. Upon administration of the nucleotide analog prodrug NUC-3373, NUC-3373 is readily taken up by tumor cells. In the tumor cell, the phosphoramidate moiety is removed and NUC-3373 is converted to its active form FUDR-MP. In turn, FUDR-MP binds to and inhibits thymidylate synthase (TS), resulting in the depletion of thymidine triphosphate (TTP) and thus DNA synthesis. With the phosphoramidate moiety attached to FUDR-MP, NUC-3373, compared to 5-FU, is more lipophilic and accumulates in cancer cells by passive diffusion and does not require a nucleoside transporter, thereby generating higher intracellular concentrations. In addition, compared to 5-FU, once inside the cell FUDR-MP does not need to be phosphorylated and is already in its active form. Unlike 5-FU, NUC-3373 does not get deactivated or converted into toxic metabolites by dihydropyrimidine dehydrogenase (DPD) and thymidine phosphorylase (TP), which leads to both a longer half-life and less toxicity. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.