| Cas No.: | 749234-11-5 |
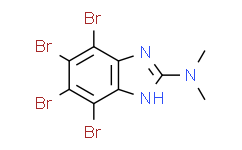
| Chemical Name: | 4,5,6,7-Tetrabromo-N,N-dimethyl-1H-benzimidazol-2-amine |
| Synonyms: | 4,5,6,7-tetrabromo-N,N-dimethyl-1H-benzimidazol-2-amine;1H-Benzimidazol-2-amine, 4,5,6,7-tetrabromo-N,N-dimethyl-;DMAT;Casein kinase II Inhibitor;CK2 Inhibitor;2-Dimethylamino-4,5,6,7-tetrabromo-1H-benzimidazole;4,5,6,7-Tetrabromo-N,N-dimethyl-1H-benzo[d]imidazol-2-amine;CK2 Inhibitor II;ck2inhibitor;CK2 Inhibitor II, DMAT;DIMETHYL-(4,5,6,7-TETRABROMO-1H-BENZOIMIDAZOL-2-YL)-AMINE;Casein Kinase II Inhibitor II, DMAT;InSolution™ Casein Kinase II Inhibitor, DMAT;1zoe;K25;CK2 Inhibitor 2;DMAT(CK2 Inhibitor);C9H7br4N3;GTPL9323 |
| SMILES: | BrC1=C(C(=C(C2=C1N([H])C(=N2)N(C([H])([H])[H])C([H])([H])[H])Br)Br)Br |
| Formula: | C9H7Br4N3 |
| M.Wt: | 476.788 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | DMAT is a potent and specific CK2 inhibitor with an IC50 value of 130 nM. |
| In Vivo: | DMAT application in vivo reduces tumor growth in a xenotransplant model by interference with tumor cell proliferation. Biochemical parameters and histology following DMAT administration revealed no alterations in liver tissue[4]. |
| In Vitro: | DMAT (1 μM-2.5 μM) DMAT is more efficient in killing antiestrogen resistant cells than parental antiestrogen sensitive MCF-7 cells. DMAT-induced cell death of antiestrogen resistant cells is mediated by caspases. DMAT inhibits CK2 activity but the inhibition is similar in the three cell lines, MCF-7, TAMR-1 and 182R-6[1]. DMAT has effects on H295R cell proliferation at concentrations of 10-4 and 10-5mol/Las compared with the control. DMAT (100 μM) significantly increases apoptosis of H295R cells. DMAT (1 nM-1 μM) significantly decreases aldosterone release into supernatants of 72-h H295R cell cultures as compared with the control[2]. DMAT also inhibits PIM1 by a mechanism which is competitive with respect to ATP, and it is a powerful inhibitor of kinases other than CK2[3]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.