| Cas No.: | 522-17-8 |
| Chemical Name: | Deguelin |
| Synonyms: | Deguelin;(7as-cis)-oxy-;3h-bis(1)benzopyrano(3,4-b:6’,5’-e)pyran-7(7ah)-one,13,13a-dihydro-9,10-dimeth;(-)-DEGUELIN;(7AS,13AS)-13,13A-DIHYDRO-9,10-DIMETHOXY-3,3-DIMETHYL-3H-BIS[1]BENZOPYRANO[3,4-B:6',5'-E]PYRAN-7(7AH)-ONE;(7aS,13aS)-13,13a-Dihydro-9,10-dimethoxy-3,3-dimethyl-(3H)-bis[1]benzopyrano[3.4-b.6'.5'-e]pyran-7(7aH)-one;12a-DEOXYTEPHROSIN;(7aS)-3,3-Dimethyl-9,10-dimethoxy-7,7aα,13,13aα-tetrahydro-3H-bis[1]benzopyrano[3,4-b:6',5'-e]pyran-7-one;(−)-Deguelin;3H-[1]Benzopyrano[3,4-b]pyrano[2,3-h][1]benzopyran-7(7aH)-one,13,13a-dihydro-9,10-dimethoxy-3,...;3H-[1]Benzopyrano[3,4-b]pyrano[2,3-h][1]benzopyran-7(7aH)-one,13,13a-dihydro-9,10-dimethoxy-3,3-dimethyl-, (7aS,13aS)-;(-)-cis-Deguelin;Tocris-1770;(7aS,13aS)-13,13a-Dihydro-9,10-dimethoxy-3,3-dimethyl-3H-bis[1]benzopyrano[3,4-b:6′,5′-e]pyran-7(7aH)-one;DEGUELIN(-);K5Z93K66IE;(-)-Deguelin, Mundulea sericea;ORDAZKGHSNRHTD-UXHICEINSA-N;Deguelin/;Spectrum_001044;3H-Bis(1)benzopyrano;Spectrum2_000298;Spectrum3_001122;Spectrum5_001852;Spectrum4_001965;3H-Bis(1);KBioSS_001524;SPECTRUM201138;KBioGR_002 |
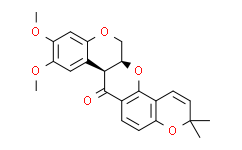
| SMILES: | O1C2C3C([H])=C([H])C(C([H])([H])[H])(C([H])([H])[H])OC=3C([H])=C([H])C=2C([C@@]2([H])C3=C([H])C(=C(C([H])=C3OC([H])([H])[C@@]12[H])OC([H])([H])[H])OC([H])([H])[H])=O |
| Formula: | C23H22O6 |
| M.Wt: | 394.4172 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Deguelin, a naturally occurring rotenoid, is known to be an Akt inhibitor and to have an anti-tumor effect on several cancers; decrease levels of phosphorylated Akt. in vitro: Deguelin in a dose and time dependent manner inhibited the growth of MDA-MB-231, MDA-MB-468, BT-549 and BT-20 cells. Deguelin administration causes a significant HNSCC cell death. Deguelin induces both cell apoptosis and autophagy by modulating multiple signaling pathways in cultured HNSCC cells. Deguelin inhibits Akt signaling, and down-regulates survivin and cyclin-dependent kinase 4 (Cdk4) expressions, by disrupting their association with heat shock protein-90 (Hsp-90). Deguelin induces ceramide production through de novo synthase pathway to promote HNSCC cell death. Importantly, increased ceramide level activates AMP-activated protein kinase (AMPK), which then directly phosphorylates Ulk1 and eventually leads to cell autophagy. Deguelin inhibited the proliferation of MPC-11 cells in a concentration- and time-dependent manner and caused the apoptotic death of MPC-11 cells. Following exposure to deguelin, the phosphorylation of Akt was decreased. The inhibition of cell growth may be associated with decreased levels of phosphorylated Akt. Deguelin-induced apoptosis was characterized by the upregulation of Bax, downregulation of Bcl-2 and activation of caspase-3. in vivo: Deguelin (2 or 4 mg/kg body weight), when injected intraperitoneally, reduced the in vivo tumor growth of MDA-MB-231 cells transplanted subcutaneously in athymic mice. Moreover it was nontoxic as evident from daily observations on mobility, food and water consumption and comparison of bodyweight and other visceral organ weights with those in control animals at the termination of the study. In the colon cancer xenograft model, the volume of the tumor treated withdeguelin was significantly lower than that of the control, and the apoptotic index for deguelin-treated mice was much higher. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.