| Cas No.: | 112093-28-4 |
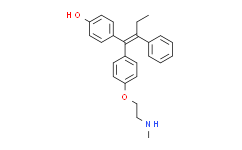
| Chemical Name: | Phenol,4-[(1Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]- |
| Synonyms: | Phenol,4-[(1Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]-;Endoxifen;4-[(Z)-1-[4-[2-(methylamino)ethoxy]phenyl]-2-phenylbut-1-enyl]phenol;Endoxifen (Z-isomer);Endoxifen NEW;4-Hydroxy-N-desmethyltamoxifen;4OHNDtam;UNII-46AF8680RC;Z-Endoxifen;4-[(1Z)-1-[4-[2-(Methylamino)ethoxy]phenyl]-2-phenyl-1-buten-1-yl]phenol;N-Desmethyl-4-hydroxytamoxifen |
| SMILES: | CNCCOC1=CC=C(C=C1)\C(C2=CC=C(O)C=C2)=C(CC)/C3=CC=CC=C3 |
| Formula: | C25H27NO2 |
| M.Wt: | 373.48700 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Endoxifen Z-isomer is the most important Tamoxifen metabolite responsible for eliciting the anti-estrogenic effects of this drug in breast cancer cells expressing estrogen receptor-alpha (ERα). Endoxifen inhibits hERG tail currents at 50 mV in a concentration-dependent manner with IC50 values of 1.6 μM.Estrogen Receptor/ERREndoxifen Z-isomer is considered a prodrug, since it has a much higher potency for the estrogen receptor than its parent drug. Endoxifen inhibits the hERG channel protein trafficking to the plasma membrane in a concentration-dependent manner with Endoxifen being more potent than Tamoxifen. [1] Endoxifen is also shown to be a more potent inhibitor of estrogen target genes when ERβ is expressed. Additionally, low concentrations of Endoxifen Z-isomer observed in Tamoxifen treated patients with deficient CYP2D6 activity (20 to 40 nM) markedly inhibit estrogen-induced cell proliferation rates in the presence of ERβ, whereas much higher Endoxifen Z-isomer concentrations are needed when ERβ is absent.[2] |
| Target: | hERG Potassium Channel IC50 value: 1.6 μM [1] |
| References: | [1]. Chae YJ, et al. Endoxifen, the active metabolite of tamoxifen, inhibits cloned hERG potassium channels. Eur J Pharmacol. 2015 Apr 5;752:1-7. [2]. Wu X, et al. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011 Mar 10;13(2):R27. |