| Cas No.: | 220127-57-1 |
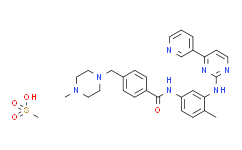
| Chemical Name: | Imatinib mesylate |
| Synonyms: | Imatinib Mesylate;4-[(4-methyl-1-piperazinyl)methyl]-n-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]phenyl]-benzamide monomethanesulfonate;4-[(4-methylpiperazin-1-yl)methyl]-n-[4-methyl-3-[(4-pyridin-3-ylpyrimidin-2-yl)amino]phenyl]benzamide methanesulfonate;CGP-57148B;GLEEVEC;GLIVEC;IMATINIB METHANESULFONATE;STI-571;IMATINIB BASE(IMA-3);Imatinib Methanesulfonate, STI-571, CGP-57148B, Glivec;IMATINIB MESYLATE (IMATINIB METHANESULFONATE);alpha-IMATINIB MESYLATE;Imatinib mesylate(TINIBS );Imatinib Mesylate (STI571);4-(4-Methylpiperazin-1-ylmethyl)-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)phenyl]benzamide methanesulfonic aci...;4-(4-Methylpiperazin-1-ylmethyl)-N-[4-methyl-3-(4-pyridin-3-yl-pyrimidin-2-ylamino)phenyl]benzamide methanesulfonic acid salt;Gleevec (Imatinib Mesylate);Imatinib;Imatinib (mesylate);Imatinib Mesilate;Imatinib Mesylate (Imatinib Methanesulfonate,Gleevec®,Glivec® );Imatinnib Mesylate;matinib mesylate;Gleevac;Imantinib mesylate;Imatinib mesylate;4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-phenyl]benzamide methanesulfonate;4-[(4-Methylpiperazin-1-yl)methyl]-N-(4-methyl-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}phenyl)benzamide mesylate;CGP 57148;Genfatinib;imatinib monomesylate;CGP 57148B;Imatinib, methanesulfonate salt;8A1O1M485B;STI 571;Imatinib mesylate, 98%;Imatinib mesylate (USAN);Imatinib mes;Imatinib mesylate [USAN];DSSTox_RID_79501;DSSTox_CID_20502;DS |
| SMILES: | S(C([H])([H])[H])(=O)(=O)O[H].O=C(C1C([H])=C([H])C(=C([H])C=1[H])C([H])([H])N1C([H])([H])C([H])([H])N(C([H])([H])[H])C([H])([H])C1([H])[H])N([H])C1C([H])=C([H])C(C([H])([H])[H])=C(C=1[H])N([H])C1=NC([H])=C([H])C(C2=C([H])N=C([H])C([H])=C2[H])=N1 |
| Formula: | C30H35N7O4S |
| M.Wt: | 589.7084 |
| Purity: | 99% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Imatinib mesylate is the mesylate salt of imatinib, a tyrosine kinase inhibitor with antineoplastic activity. Imatinib binds to an intracellular pocket located within tyrosine kinases (TK), thereby inhibiting ATP binding and preventing phosphorylation and the subsequent activation of growth receptors and their downstream signal transduction pathways. This agent inhibits TK encoded by the bcr-abl oncogene as well as receptor TKs encoded by the c-kit and platelet-derived growth factor receptor (PDGFR) oncogenes. Inhibition of the bcr-abl TK results in decreased proliferation and enhanced apoptosis in malignant cells of Philadelphia-positive (Ph+) hematological malignancies such as CML and ALL; effects on c-kit TK activity inhibit mast-cell and cellular proliferation in those diseases overexpressing c-kit, such as mastocytosis and gastrointestinal stromal tumor (GIST). Check For active clinical trials or closed clinical trials using this agent. (NCI Thesaurus). For the detailed information about the solubility of Imatinib mesylate in water, the solubility of Imatinib mesylate in DMSO, the solubility of Imatinib mesylate in PBS buffer, the animal experiment(test) of Imatinib mesylate, the in vivo,in vitro and clinical trial test of Imatinib mesylate, the cell experiment(test) of Imatinib mesylate, the IC50, EC50 and Affinity of Imatinib mesylate , please contact DC Chemicals. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.