| Cas No.: | 72926-24-0 |
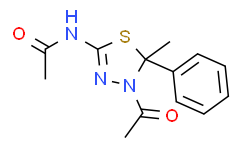
| Chemical Name: | N-(4-acetyl-5-methyl-5-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)a Cetamide |
| Synonyms: | N-(4-Acetyl-5-methyl-5-phenyl-4,5-dihydro-1,3,4-thiadiazol-2-yl)a cetamide;2,4,6-Cycloheptatrien-1-one, 3-acetyl-5,7-dibromo-2-hydroxy-;3-acetyl-5,7-dibromotropolone;ACMC-20mef0;AGN-PC-00O3CS;3-acetyl-5-acetylamino-2-methyl-2-phenyl-2,3-dihydro-[1,3,4]thiadiazole;CTK0G1775;K-858;K858;N-(4-Acetyl-4,5-dihydro-5-methyl-5-phenyl-1,3,4-thiadia zol-2-yl)acetamide;N-(4-Acetyl-4,5-dihydro-5-methyl-5-phenyl-1,3,4-thiadiazol-2-yl)acetamide;K858 (Racemic) |
| SMILES: | CC(NC1=NN(C(C)=O)C(C2=CC=CC=C2)(C)S1)=O |
| Formula: | C13H15N3O2S |
| M.Wt: | 277.3421 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | K858 Racemic is an ATP-uncompetitive inhibitor of Eg5 with an IC50 of 1.3 μM. |
| In Vivo: | K858 (50, 150 mg/kg, p.o.) shows antitumor activity in an A2780 ovarian cancer xenograft model, also inhibits tumor grwoth in a HCT116 colon cancer xenograft model via 100 mg/kg twice a day orally for 5 days. K858 (100 mg/kg, p.o., qd ×5) displays no neurotoxic side effects in mice[1]. |
| In Vitro: | K858 Racemic is an ATP-uncompetitive inhibitor of Eg5 with an IC50 of 1.3 μM. K858 does not inhibit the ATPase activity of the mitotic kinesins CENP-E and MKLP1, or the conventional kinesin heavy chain even at 200 μM. K858 induces mitotic arrest and growth inhibition via the activation of the Mad2-mediated spindle checkpoint. K858 (5 μM) induces mitotic cell death in cancer cells but not in normal cells[1]. K858 (1, 10, 100 μM) inhibits the MCF7, BT474 and SKBR3 cell lines, and only at 10 and 100 μM suppresses MDA-MB231 cell line after treatment for 24 h. K858 incereases Bax/Bcl2 RNA ratio and survivin in the four cell lines. Furthermore, the up-regulation of survivin is totally reversed by wortmannin (phosphoinositide 3-kinase AKT) in MCF7 cells[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.