| In Vitro: |
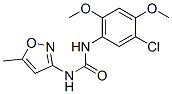
PNU-120596 increases agonist (Ach)-evoked calcium flux mediated by an engineered variant of the human α7 nAChR. PNU-120596 increases agonists (choline and ACh)-evoked currents mediated by wild-type receptors and also demonstrates a pronounced prolongation of the evoked response in the continued presence of agonist in Xenopus oocytes. PNU-120596 increases the channel mean open time ofα7 nAChRs but has no effect on ion selectivity and relatively little, if any, effect on unitary conductance. When applied to acute hippocampal slices, PNU-120596 increases the frequency of ACh-evoked GABAergic postsynaptic currents measured in pyramidal neurons; this effect is suppressed by TTX, suggesting that PNU-120596 modulates the function of α7 nAChRs located on the somatodendritic membrane of hippocampal interneurons [1]. Besides the positive modulation to α7 nAChR, PNU-120596 induces a profound retardation of the kinetics of desensitization, raising the potential of Ca2+-induced toxicity through excessive stimulation of α7 nAChR [2]. PNU-120596 causes changes in cysteine accessibility at the inner beta sheet, transition zone and agonist binding site while binding to α7 nAChR. Binding sites for PNU-120596 are not in the agonist-binding sites and PNU-120596 enhances agonist-evoked gating of nicotinic receptors by eliciting conformational effects that are similar but nonidentical to the gating conformations promoted by Ach [3] |