| Cas No.: | 182485-36-5 |
| Chemical Name: | Phosphinic acid,P-methyl-P-(1,2,3,6-tetrahydro-4-pyridinyl)- |
| Synonyms: | Phosphinic acid,P-methyl-P-(1,2,3,6-tetrahydro-4-pyridinyl)-;(1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinic acid hydrate;TPMPA;(1,2,5,6-Tetrahydropyridin-4-yl)methylphosphinicacid;TPMPA hydrate;tpmpa hydrate;MFUKVPOVVKKLRQ-UHFFFAOYSA-N;(1,2,5,6-TETRAHYDROPYRIDIN-4-YL)METHYLPHOSPHINIC ACID;(1,2,5,6-TETRAHYDROPYRIDIN-4-YL)METHYLPHOSPHINIC ACID HYDRATE |
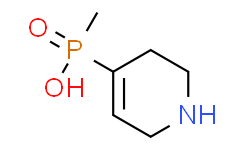
| SMILES: | CP(C1=CCNCC1)(=O)O |
| Formula: | C6H12NO2P |
| M.Wt: | 161.138742446899 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | TPMPA, a hybrid of isoguvacine and 3-APMPA, is the first selective antagonist for a GABAC receptor (KB = 2.1 μM), but not to interact with GABAA (KB = 320 μM) or GABAB receptors (EC50 = 500 μM). TPMPA has the potential for the research of suppressing orientation selectivity in ganglion cells[1][2][3]. |
| Target: | KB: 2.1 μM (GABAC) |
| In Vitro: | TPMPA antagonizes the GABA currents of ρ1 receptors (IC50 = 1.6 μM) and those of the chimeric ρ1/α1 receptors with approximately the same potency (IC50 = 1.3 μM)[1]. TPMPA shows weak activity against rho-1 and rho-2 receptors, with the KB values of 2.0 and 15.6 μM, respectively[2] |
| References: | [1]. Martínez-Torres A, et al. GABArho 1/GABAAalpha 1 receptor chimeras to study receptor desensitization. Proc Natl Acad Sci U S A. 2000;97(7):3562-3566. [2]. Graham A. R. Johnston, et al. Neurologically-active compounds. WO1998058939A1 [3]. Johnston GA. GABAc receptors: relatively simple transmitter -gated ion channels?. Trends Pharmacol Sci. 1996;17(9):319-323. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.