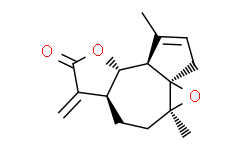
| Cas No.: | 84692-91-1 |
| Chemical Name: | Arglabin |
| Synonyms: | 3H-Oxireno[8,8a]azuleno[4,5-b]furan-8(4aH)-one,5,6,6a,7,9a,9b-hexahydro-1,4a-dimethyl-7-methylene-, (3aR,4aS,6aS,9aS,9bR)-;Arglabin;(+)-Arglabin;3H-Oxireno[8,8a]azuleno[4,5-b]furan-8(4aH)-one, 5,6,6a,7,9a,9b-hexahydro-1,4a-dimethyl-7-methylene-, (3aR,4aS,6aS,9aS,9bR)-;3H-?Oxireno[8,?8a]?azuleno[4,?5-?b]?furan-?8(4aH)?-?one, 5,?6,?6a,?7,?9a,?9b-?hexahydro-?1,?4a-?dimethyl-?7-?methylene-?, (3aR,?4aS,?6aS,?9aS,?9bR)?-;(3aR,4aS,6aS,9aS,9bR)-5,6,6a,7,9a,9b-Hexahydro-1,4a-dimethyl-7-methylene-3H-oxireno[8,8a]azuleno[4,5-b]furan-8(4aH)-one;1(R),10(S)-Epoxy-5(S),5(S),7(S)-guaia-3(4),11(13)-dien-6,12-olide;(1R,3S,6R,10R,11R)-3,12-Dimethyl-7-methylidene-2,9-dioxatetracyclo[9.3.0.01,3.06,10]tetradec-12-en-8;(1S,3R)-3,12-dimethyl-7-methylidene-2,9-dioxatetracyclo[9.3.0.01,3.06,10]tetradec-12-en-8-one |
| SMILES: | O1[C@]2(C([H])([H])[H])C([H])([H])C([H])([H])C3([H])C(=C([H])[H])C(=O)OC3([H])C3([H])C(C([H])([H])[H])=C([H])C([H])([H])[C@@]123 |
| Formula: | C15H18O3 |
| M.Wt: | 246.3016 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | AR-M 1000390 hydrochloride is an exceptionally selective, potent δ opioid receptor agonist with an EC50 of 7.2±0.9 nM for δ agonist potency. |
| In Vivo: | Rats are treated with 5, 100, and 600 μmol/kg of AR-M 1000390 (AR-M100390) for 3 and/or 7 days; another group of rats treated with 600 μmol/kg of compound are allowed to recover for 14 days. AR-M 1000390 (600 μmol/kg) causes vacuolation in the β-cell of the rat pancreas that is associated with depletion of insulin and hyperglycemia after 7 days of dosing. Treatment of rats with 600 μmol/kg of AR-M 1000390 results in vacuolation of the β-cell of the rat pancreas that is similar to that reported for cyclizine and cyproheptadine[2]. |
| In Vitro: | AR-M 1000390 (Compound 6a) exhibits the binding affinities (IC50) of 0.87±0.23 nM for the δ opioid receptor and extremely high selectivity over the µ receptor (IC50=3800±172 nM) and the κ receptor (IC50=7470±606 nM)[1]. RINm5F cells are treated with AR-M 1000390 (AR-M100390) and Cyclizine for 16-24 h before measurement of intracellular and secreted insulin levels. AR-M 1000390 mediates a dose-dependent decrease in insulin content with a maximal inhibition of ~90% at the highest concentration tested (10 μM)[2]. |
| Cell Assay: | RINm5F cells are seeded in 24-well plates and treated with vehicle (DMSO), 10 μM AR-M 1000390 (AR-M100390), and 10 μM Cyclizine in serum-free medium; cells are rinsed with phosphate-buffered saline and stored at -80°C until analysis. RNA is isolated with the RNeasy purification system with DNAse treatment[2]. |
| Animal Administration: | Rats[2] Han Wistar rats (six per treatment group) are treated with vehicle (saline) or 5, 100, and 600 μmol/kg/day of AR-M 1000390 (AR-M100390) for 7 days. A separate group of rats are treated with 600 μmol/kg/day for 7 days followed by a 14-day recovery period. Another group is treated with 600 μmol/kg/day for 3 days. Blood sampling for glucose, lipids, and insulin measurements are taken on days 2, 4, 8, and 22. Blood sampling for AR-M 1000390 concentration measurements are collected on days 4 and 8. The animals are euthanized with CO2 on days 4, 8, and 22 and the pancreas isolated and processed for histopathology, insulin immunohistochemistry, and insulin mRNA analyses[2]. |
| References: | [1]. Wei ZY, et al. N,N-Diethyl-4-(phenylpiperidin-4-ylidenemethyl)benzamide: a novel, exceptionally selective, potent delta opioid receptor agonist with oral bioavailability and its analogues. J Med Chem. 2000 Oct 19;43(21):3895-905. [2]. Otieno MA, et al. Mechanistic investigation of N,N-diethyl-4-(phenyl-piperidin-4-ylidenemethyl)-benzamide-inducedinsulin depletion in the rat and RINm5F cells. Toxicol Sci. 2008 Sep;105(1):221-9. |