| Cas No.: | 18080-67-6 |
| Chemical Name: | 2,3-Bis(bromomethyl)quinoxaline 1,4-dioxide |
| Synonyms: | 2,3-Bis(bromomethyl)quinoxaline 1,4-dioxide;2,3-bis(bromomethyl)-4-oxidoquinoxalin-1-ium 1-oxide;Conoidin A;2,3-Bis-brommethyl-chinoxalin-1,4-dioxid |
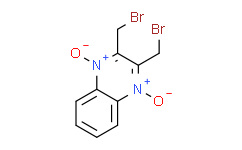
| SMILES: | BrCC1C(CBr)=[N+]([O-])C2C(=CC=CC=2)[N+]=1[O-] |
| Formula: | C10H8Br2N2O2 |
| M.Wt: | 347.990720748901 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Conoidin A is a cell permeable inhibitor of T. gondii enzyme peroxiredoxin II (TgPrxII) with nematicidal properties. Conoidin A covalently binds to the peroxidatic Cys47 of TgPrxII, irreversibly inhibiting its hyperperoxidation activity with an IC50 of 23 µM. Conoidin A also inhibits hyperoxidation of mammalian PrxI and PrxII (but not PrxIII)[1][2]. Conoidin A has antioxidant, neuroprotective effects and can be used for the research of ischaemic heart disease[3]. |
| Target: | IC50: 23 µM (T. gondii enzyme peroxiredoxin II (TgPrxII))[1] |
| In Vivo: | Conoidin A (intraperitoneal injection; 5 mg/kg; for three successive days before MI/R injury) blocks the effect of Luteolin (HY-N0162) on the ST‐segment elevation. Furthermore, an increase in the infarct size presented of the MI/R group can be reduced by Luteolin. But pre‐treatment with conoidin A abolishs the effect of Luteolin. Pre‐treatment with conoidin A also prevents Luteolin-reduced activities of CK‐MB, AST and LDH in vivo[3]. Animal Model: Rat myocardial I/R model[3] Dosage: 5 mg/kg Administration: Intraperitoneal injection; 5 mg/kg; for three successive days before MI/R injury Result: Significantly reversed the antioxidative effect of Luteolin. Impaired the protective effects of luteolin. |
| In Vitro: | Peroxiredoxins are a widely conserved family of enzymes that function in antioxidant defense and signal transduction. And the changes in PrxII expression are associated with a variety of human diseases, including cancer[1]. Conoidin A binds to the peroxidatic cysteine of TgPrxII, inhibiting its enzymatic activity in vitro. Conoidin A also shown to alkylate or crosslink catalytic cysteines of wild type AcePrx-1 in Ancylostoma ceylanicum and human PrxII and PrxIV with similar efficiency. But it is ineffective to mitochondrial hPrxIII[2]. Conoidin A (5 µM) can inhibit the glucose oxidase-mediated hyperoxidation of mammalian peroxiredoxin I and II[2]. |
| References: | [1]. Jeralyn D Haraldsen, et al. IDENTIFICATION OF CONOIDIN A AS A COVALENT INHIBITOR OF PEROXIREDOXIN II. Org Biomol Chem. 2009;7:3040-3048. [2]. Gu Liu, et al. Optimisation of conoidin A, a peroxiredoxin inhibitor. ChemMedChem. 2010 Jan;5(1):41-5. [3]. Bo Wei, et al. Luteolin ameliorates rat myocardial ischaemia-reperfusion injury through activation of peroxiredoxin II. Br J Pharmacol |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.