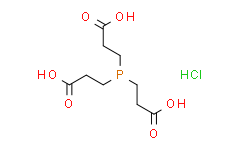
| Cas No.: | 51805-45-9 |
| Chemical Name: | 3,3',3''-Phosphinetriyltripropanoic acid hydrochloride |
| Synonyms: | 3,3',3''-Phosphinetriyltripropanoic acid hydrochloride;3,3',3''-PHOSPHINETRIYL-TRIPROPIONIC ACID HYDROCHLORIDE;Tris(2-carboxyethyl)phosphine hydrochloride;TCEP;Tris(2-carboxyethyl)phosphine, HCl;Tris(2-carboxyethyl)phosphine;TCEP Hydrochloride;TCEP HCl;TCEP (hydrochloride);3-[bis(2-carboxyethyl)phosphanyl]propanoic acid,hydrochloride |
| SMILES: | Cl.P(CCC(O)=O)(CCC(O)=O)CCC(O)=O |
| Formula: | C9H16ClO6P |
| M.Wt: | 286.646503448486 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | TCEP hydrochloride (Tris(2-carboxyethyl)phosphine hydrochloride) is a non-thiol reducing agent that is more stable and produces a faster S-S reductive reaction than other chemical reductants. TCEP hydrochloride is a trialkylphosphine, selectively reduces protein disuldes without altering the properties or interacting with thiol-directed agents in the reaction mixture. TCEP hydrochloride is also a commonly used reducing agent in the DNA/AuNP chemistry[1][2][3][4]. |
| In Vitro: | TCEP hydrochloride has been introduced which oers the prospect of serving as an alternative to the more commonly employed DTT in the NF-κB-DNA binding reactions in vitro, using recombinant p50 protein and a 32P-labelled κB oligonucleotide. DTT promotes NF-κB-DNA binding in concentrations from 0.25 to 2.6 mM in binding reactions. However, in the presence of 0.25 mM DTT, inhibition of NF-κB binding is seen only at Hg2+ concentrations greater than 100 μM and results are highly variable. In contrast, TCEP hydrochloride promotes NF-κB-DNA binding in a dose-related manner in concentrations from 0.25 to 6 mM. In the presence of even 6 mM TCEP hydrochloride, Hg2+ prevents NF-κB-DNA binding at concentrations as low as 20 μM in binding reactions[1]. The human lactoferrin (hLF) peptide is dissolved in phosphate buffer to a concentration of 0.1 mm. Reduction of the disulfide bonds is obtained by adding a 30-fold molar excess of TCEP hydrochloride with subsequent incubation for 2 h at 37 ℃[2]. |
| References: | [1]. Dieguez-Acuña FJ, et al. Inhibition of NF-kappaB-DNA binding by mercuric ion: utility of the non-thiol reductant, tris(2-carboxyethyl)phosphine hydrochloride (TCEP), on detection of impaired NF-kappaB-DNA binding by thiol-directed agents. Toxicol In Vitro. 2000 Feb;14(1):7-16. [2]. Duchardt F, et al. A cell-penetrating peptide derived from human lactoferrin with conformation-dependent uptake efficiency. J Biol Chem. 2009 Dec 25;284(52):36099-108. [3]. Sequeira MA, et al. Modulating amyloid fibrillation in a minimalist model peptide by intermolecular disulfide chemical reduction. Phys Chem Chem Phys. 2019 Jun 5;21(22):11916-11923. [4]. Wu R, et al. Effects of Small Molecules on DNA Adsorption by Gold Nanoparticles and a Case Study of Tris(2-carboxyethyl)phosphine (TCEP). Langmuir. 2019 Oct 15;35(41):13461-13468. |