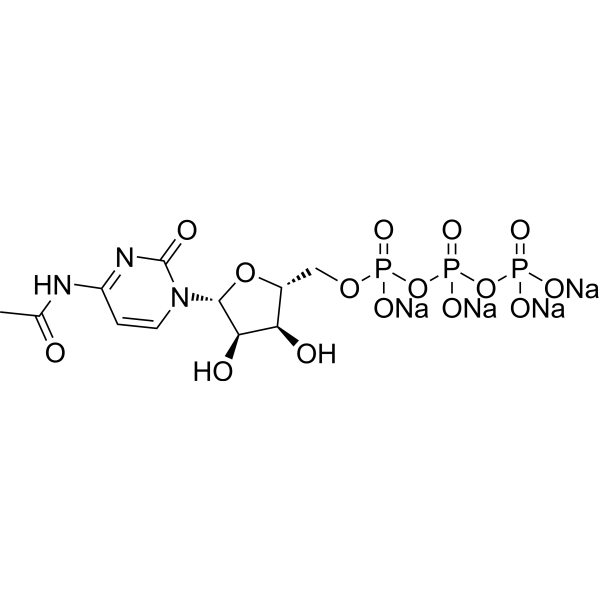| DC60788 |
PT-129
Featured
|
PT-129 is a potent G3BP1/2 PROTAC degrader and dissolves preformed stress granules (SG). PT-129 disrupts the protective SG environment, making cancer cells more susceptible to stress-induced cell death. |

|
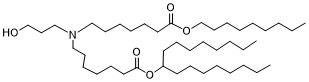
| DC65387 |
BP Lipid 132(LP01 analog)
Featured
|
BP Lipid 132 is a well-designed ionizable lipid that combines effective mRNA encapsulation with enhanced biodegradability and tissue clearance, making it a valuable component in LNP-based mRNA delivery systems. |
|
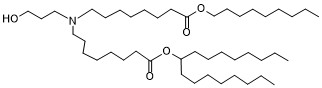
| DC65390 |
BP Lipid 135
Featured
|
BP Lipid 135 is a well-designed ionizable lipid optimized for mRNA encapsulation and delivery. Its propanolamine headgroup, ester bonds at the C8 position, and 9-carbon tail contribute to efficient mRNA complexation, stability during delivery, and improved biodegradability. These properties make it a valuable component in LNPs for gene therapy and other mRNA-based therapeutic applications. |
|
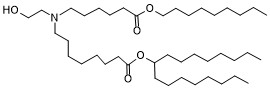
| DC65362 |
BP Lipid 114
Featured
|
BP Lipid 114 is a well-designed ionizable lipid optimized for mRNA encapsulation and delivery. Its ethanolamine headgroup, ester bonds at the C6 and C8 positions, and 9-carbon tail contribute to efficient mRNA complexation, stability during delivery, and improved biodegradability. These properties make it a valuable component in LNPs for gene therapy and other mRNA-based therapeutic applications. |
|
| DC60789 |
SM-86 Analog-1
Featured
|
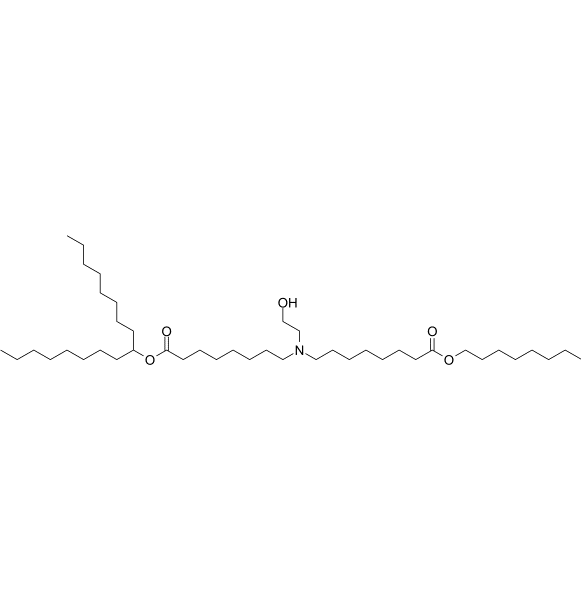
SM-86 Analog-1 is a novel ionizable lipid designed to improve the delivery of RNA via lipid nanoparticles (LNPs) It is derived from SM-86,with 8 carbon within its hydrophobic tail. |
|
| DC60790 |
DesMEM AZD4694
Featured
|
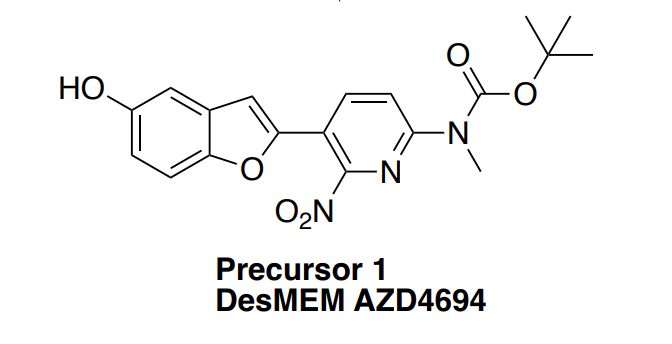
DesMEM AZD4694(AZD4694 Precursor 1)is the precursor of [18F] AZD4694 for the synthesis of [18F] AZD4694, an amyloid-β imaging ligand with high affinity for amyloid-β plaques. |
|
| DC60791 |
Cholestify Precursor
Featured
|
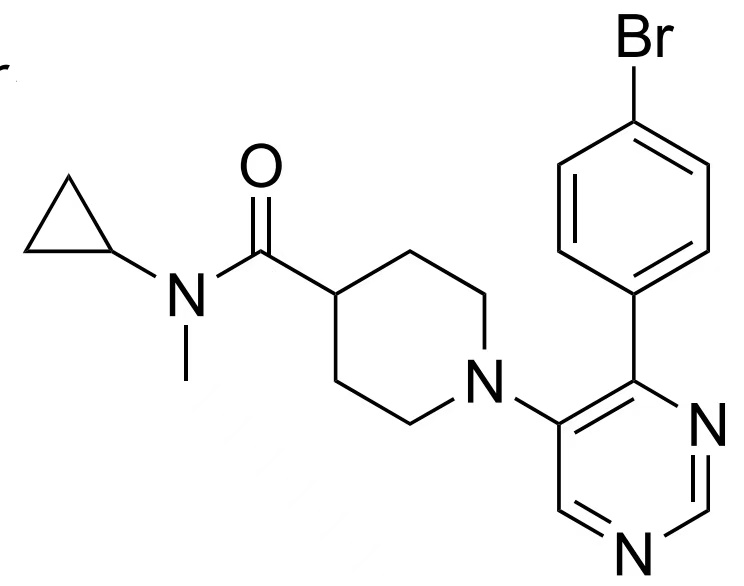
Precursor of Cholestify,18F-Cholestify, which binds cytochrome P450 46A1, detected cholesterol breakdown in the mammalian brain. CYP46A1 converts cholesterol to 24-hydroxycholesterol, a form easily eliminated from the brain. |
|
| DC67281 |
BNT-51
Featured
|
|
|
| DC67284 |
NOTA-NOC
Featured
|
|
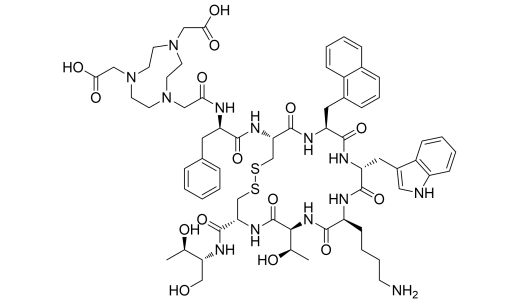
|
| DC67285 |
DOTA-Octreotide
Featured
|
|
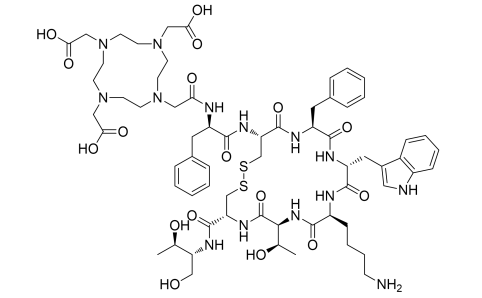
|
| DC67286 |
N-Desmethyl Galanthamine
Featured
|
N-Desmethyl Galanthamine is indeed a metabolite of Galanthamine, a well-known acetylcholinesterase (AChE) inhibitor. Galanthamine is a natural alkaloid originally derived from plants such as Galanthus (snowdrop) and is widely used in the treatment of Alzheimer's disease and other cognitive disorders due to its ability to enhance cholinergic neurotransmission. |

|
| DC67287 |
3-Methoxy-2',4',6',4-tetrahydroxychalcone
Featured
|
|
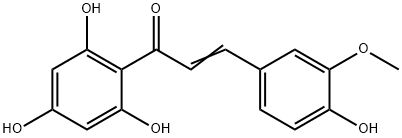
|
| DC67288 |
Oxypalmatine
Featured
|
Oxypalmatine is a bioactive alkaloid compound isolated from Phellodendron amurense, a plant commonly known as Amur cork tree. Phellodendron amurense is a traditional medicinal plant widely used in East Asian medicine, particularly in China, Japan, and Korea, for its anti-inflammatory, antimicrobial, and antipyretic properties. Oxypalmatine is one of the many alkaloids found in this plant, contributing to its pharmacological effects. |
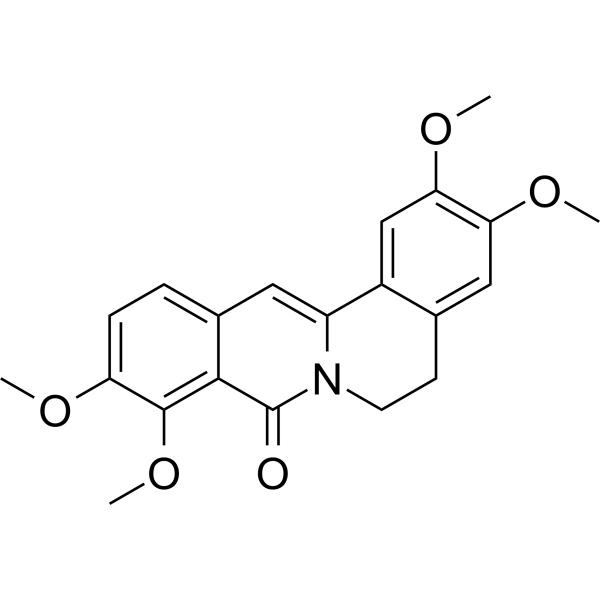
|
| DC67289 |
Hydrangetin
Featured
|
Hydrangetin is a bioactive compound that has been identified as having antiplatelet aggregation properties, meaning it can help prevent blood clots by inhibiting the clumping together of platelets. This compound can be isolated from Zanthoxylum schinifolium, a plant commonly known as the Sichuan pepper or Korean pepper, which is used in traditional medicine and culinary practices in East Asia. |
|
| DC60792 |
10-Oxo-12(Z)-octadecenoic acid
Featured
|
10-oxo-12(Z)-Octadecenoic acid is a metabolite of linoleic acid and an activator of transient receptor potential vanilloid 1 (TRPV1). It is formed from linoleic acid by conjugated linoleic acid dehydrogenase (CLA-DH) via a 10-hydroxy-12(Z)-octadecenoic acid intermediate and can also be produced from linoleic acid by gut microbiota.1 10-oxo-12(Z)-Octadecenoic acid (100 µM) selectively increases calcium levels in HEK293 cells expressing TRPV1 over those expressing TRPV2, TRPV3, TRPV4, and TRP melastatin 8 (TRPM8). It also induces inward currents in HEK293 cells expressing TRPV1, an effect that can be blocked by the TRPV1 antagonist capsazepine (Item No. 10007518). Dietary administration of 10-oxo-12(Z)-octadecenoic acid (0.1% w/w) reduces weight gain and adipose tissue weight and increases the expression of the gene encoding mitochondrial uncoupling protein 1 (Ucp1) in wild-type, but not Trpv1 knockout, mice fed a high-fat diet. It also decreases plasma glucose and triglyceride levels in diabetic KKAy mice fed a high-fat diet. |
|
| DC60793 |
LUMI-6
Featured
|
LUMI-6, a brominated ionizable lipid developed through xAI’s LUMI-lab platform, features a unique molecular design with a bromine atom critical for enhancing mRNA delivery, outperforming its debrominated derivative, LUMI-6D. In murine models, it achieved a groundbreaking 20.3% gene editing efficiency in lung epithelial cells via inhaled delivery of CRISPR-Cas9 lipid nanoparticles (LNPs), surpassing previous records and the commonly used SM-102 LNP in pulmonary Cas9 mRNA/gRNA complex delivery. In human bronchial epithelial (HBE) cells, LUMI-6 demonstrated 1.8-fold higher mRNA transfection efficiency than LUMI-6D while maintaining comparable cytotoxicity to non-brominated lipids. Its brominated tail structure optimizes mRNA encapsulation and release, balancing high efficiency with low toxicity. Notably, LUMI-6 exhibits cellular selectivity, preferentially transfecting lung epithelial cells—such as ciliated (α-tubulin+) and club (CCSP+) cells—over endothelial cells, making it highly relevant for airway-targeted therapies like cystic fibrosis and surfactant disorders. Effective for both mRNA delivery and CRISPR-Cas9 systems, LUMI-6 represents a significant advancement in pulmonary gene therapy, addressing unmet needs in treating congenital lung diseases through epithelial cell-specific editing. As the first reported LNP to achieve over 20% editing efficiency in lung epithelium via inhalation, it underscores the power of AI-driven lipid discovery in accelerating therapeutic innovation. |
|
| DC67293 |
D-Val-Gly-Arg-pNA
Featured
|
D-Val-Gly-Arg-p-nitroaniline (D-VGR-pNA) is a synthetic chromogenic peptide substrate specifically designed for assessing the enzymatic activity of tissue plasminogen activator (tPA), including its isoforms tPA I and tPA II. Upon cleavage by tPA, the release of p-nitroaniline (pNA) generates a measurable colorimetric signal, enabling precise quantification of amidolytic activity. This substrate is widely utilized in biochemical assays to study tPA’s role in fibrinolysis and to evaluate its enzymatic kinetics in both research and diagnostic applications. |
|
| DC60794 |
SABA1
Featured
|
SABA1 is an antibacterial agent effective against Pseudomonas aeruginosa and Escherichia coli. It works by inhibiting biotin carboxylase (BC), an enzyme that catalyzes the first step of the acetyl-CoA carboxylase (ACC) reaction, a process essential for bacterial fatty acid synthesis. What makes SABA1 particularly notable is its atypical inhibition mechanism: it binds to the biotin binding site of BC in the presence of ADP. This unique mode of action distinguishes SABA1 as a promising candidate for the development of new antibiotics, offering a potential solution to the growing challenge of antimicrobial resistance. |
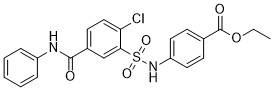
|
| DC60795 |
Boscalid
Featured
|
Boscalid is a broad-spectrum fungicide widely used in agriculture to protect crops from fungal diseases. Its bioactivity stems from its ability to inhibit fungal respiration by targeting succinate dehydrogenase (Complex II) in the mitochondrial electron transport chain. By binding to this enzyme, Boscalid blocks electron transfer, disrupting ATP synthesis and energy production in fungal cells, ultimately leading to their death. This mechanism makes Boscalid highly effective against a wide range of fungal pathogens, including Botrytis, Alternaria, Sclerotinia, and powdery mildew species. |
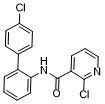
|
| DC67295 |
Lipid MK16
Featured
|
MK16 is a novel blood-brain barrier (BBB)-crossing lipid nanoparticle (BLNP) platform developed for efficient mRNA delivery to the central nervous system (CNS). Designed through structure-guided optimization, MK16 lipids integrate a γ-secretase inhibitor-derived BBB-penetrating module (MK-0752) with ionizable amino lipids, enabling systemic mRNA transport into the brain.MK16 was selected from a library of 72 BBB-crossing lipids synthesized by conjugating small-molecule BBB transporters (e.g., L-DOPA, D-serine, MK-0752) with lipid tails. Structural refinements, including acetal-functionalized alkyl chains and optimized lipid-to-mRNA ratios (12.5:1 w/w), enhanced brain delivery efficiency. MK16 BLNPs exhibit a particle size of ~137 nm, 85% mRNA encapsulation, and a pKa of 6.7–6.9, facilitating endosomal escape.MK16 BLNPs cross the BBB via caveolae- and γ-secretase-mediated transcytosis, as validated by inhibitor studies. In mice, intravenous MK16 BLNPs delivered mRNA broadly to neurons (7.4% GFP+), astrocytes (9.7% GFP+), and brain endothelial cells (9.2% GFP+), outperforming FDA-approved LNPs (MC3, SM-102) by 6–8-fold in brain luminescence. Functional studies in Ai14 mice demonstrated Cre mRNA-mediated tdTomato activation across the hippocampus, thalamus, and cortex, with triple dosing doubling transfection efficiency.Cocaine Addiction: MK16-delivered ΔFosb mRNA enhanced cocaine-conditioned place preference in mice, mimicking addiction-related neural plasticity.
Glioblastoma (GBM): Systemic MK16-Pten mRNA inhibited orthotopic U-118MG tumor growth, achieving 70% survival at 120 days vs. controls.
Human Translation: Ex vivo human brain slices showed ΔFOSB expression in neurons (4.0%) and astrocytes (6.5%), confirming clinical potential.MK16 BLNPs exhibited minimal toxicity in multi-dose regimens, with normal blood biomarkers, cytokine levels, and histopathology. Unlike MK-0752, MK16 did NOT alter NOTCH pathway genes, mitigating off-target risks. |
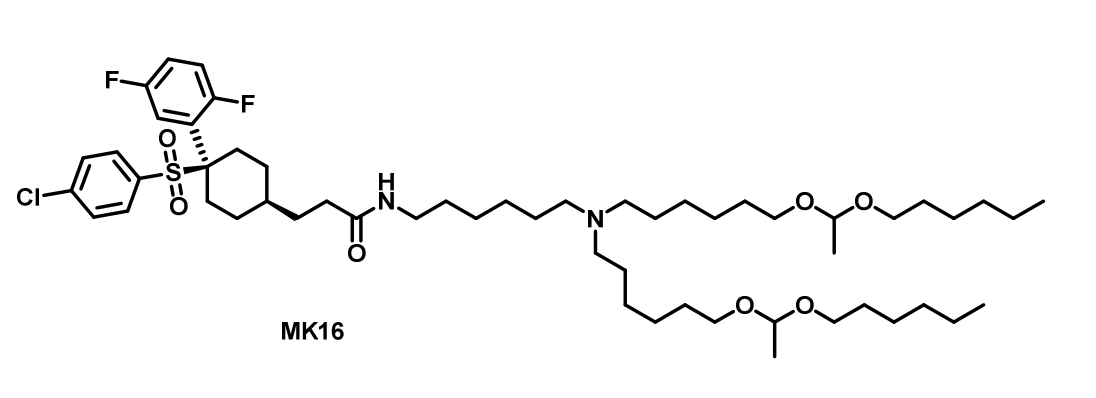
|
| DC60796 |
HIFN
Featured
|
HIFN (2-fluoro-N-(2-(5-hydroxy-1H-indol-3-yl)ethyl)nicotinamide) is a synthetic small-molecule agonist of the tropomyosin-related kinase B (TrkB) receptor, designed to mimic brain-derived neurotrophic factor (BDNF) signaling. Structurally, HIFN replaces the six-membered lactam ring of its parent compound HIOC with a fluoropyridine moiety, rendering it achiral and configurationally stable. This modification enhances binding affinity and pharmacokinetic properties.
HIFN activates TrkB by inducing receptor dimerization and phosphorylation, triggering downstream survival pathways (PI3K/Akt, MAPK/Erk). In vitro, HIFN outperforms HIOC in TrkB activation (10 nM concentration) in primary neurons and NIH-3T3-TrkB cells. In vivo, systemic administration of HIFN (30–40 mg/kg) mitigates blast-induced retinal ganglion cell (RGC) degeneration and preserves visual function (contrast sensitivity, acuity) in mice for up to 8 weeks post-injury. Its effects are TrkB-dependent, as co-treatment with the TrkB antagonist ANA-12 abolishes neuroprotection.
HIFN exhibits a critical therapeutic window of ≤3 hours post-injury and dose-dependent efficacy, with no toxicity observed at 600 mg/kg (acute) or 40 mg/kg/day (40-day chronic). Safety assessments reveal no histopathological or biochemical abnormalities in vital organs.
By combining potent TrkB activation, blood-retina barrier penetration, and a robust safety profile, HIFN emerges as a promising therapeutic candidate for traumatic optic neuropathy and broader CNS disorders involving TrkB dysregulation. |

|
| DC67296 |
Galnac GLS-15
Featured
|
GLS-15 is a novel GalNAc-derived small molecule structure designed for siRNA delivery. |
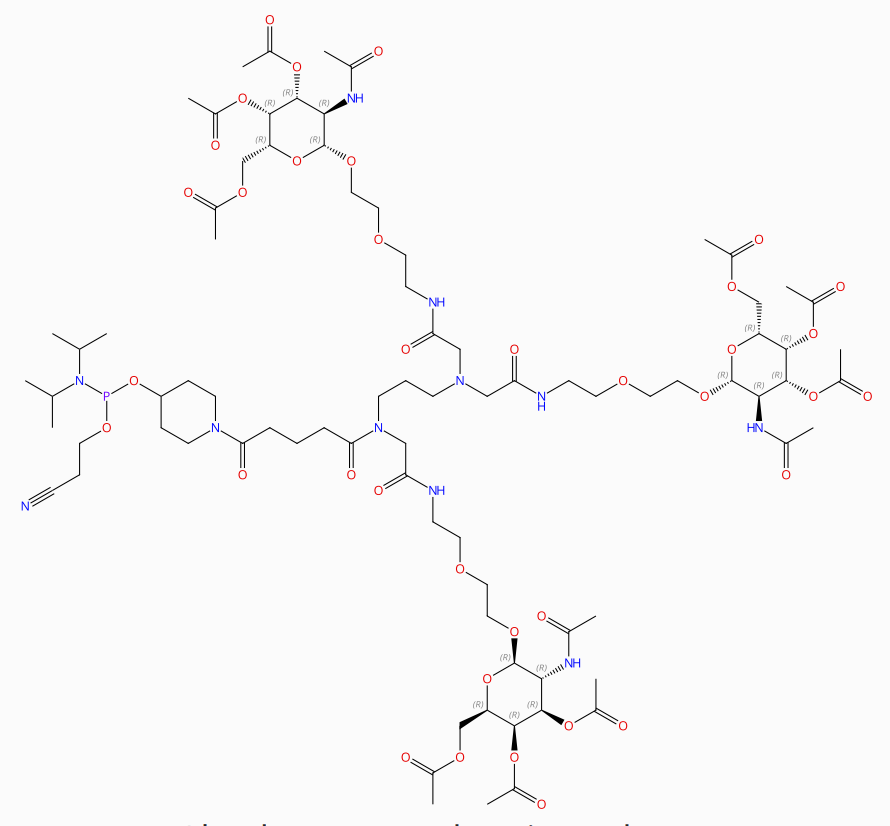
|
| DC67297 |
SM6.1 (αvβ6 ligand)
Featured
|
SM-6.1 is a small molecule delivery vehicle developed by Arrowhead, targeting αvβ6, which selectively delivers therapeutic siRNA to lung epithelial cells and mediates durable gene silencing post-inhalation. |
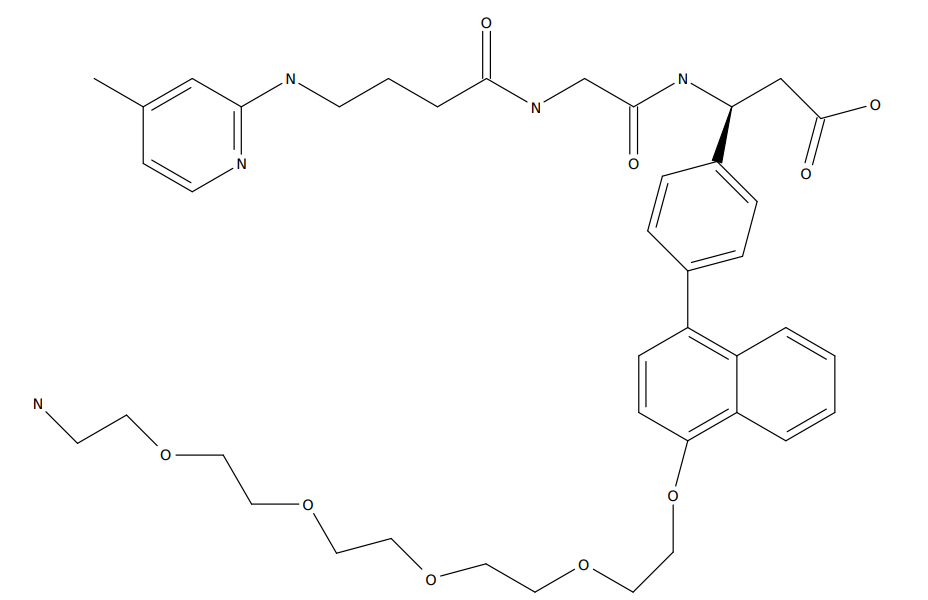
|
| DC67298 |
Lipid 5D8
Featured
|
Lipid 5D8 is a novel biodegradable ionizable lipid (IL) developed through a combinatorial chemistry strategy to overcome the limitations of conventional lipid nanoparticles (LNPs) in mRNA delivery. Synthesized via a one-step, solvent-free Michael addition reaction between amine and thiol monomers, 5D8 features asymmetric lipid tails and a biodegradable ester backbone, ensuring both structural versatility and reduced toxicity. In preclinical studies, 5D8-based LNPs demonstrated exceptional liver-targeting efficiency and mRNA delivery performance. A single intravenous dose (1 mg/kg) achieved 61% CRISPR-Cas9-mediated editing of the TTR gene in mice, reducing serum TTR protein by 90%, outperforming benchmark lipids like C12-200 (51% editing). Moreover, 5D8 enabled efficient delivery of base editors (ABE8.8 and CBE4max), achieving 42% PCSK9 editing (74% serum protein reduction) and correcting hereditary tyrosinemia in mice, significantly extending survival. Beyond gene editing, 5D8 LNPs effectively delivered siRNA (complete serum TTR clearance at 0.05 mg/kg) and enhanced hepatocyte targeting by enriching apolipoprotein E on particle surfaces. Crucially, 5D8 exhibited superior biocompatibility with no hepatotoxicity (normal ALT/AST levels), contrasting traditional LNPs. Its rapid biodegradability and "plug-and-play" design make 5D8 a versatile platform for mRNA therapeutics, holding broad potential for treating genetic disorders, cardiovascular diseases, and beyond. This innovation represents a critical advancement toward safer, high-efficiency clinical translation of gene-editing therapies.L |

|
| DC67299 |
N4-Acetylcytidine triphosphate sodium
Featured
|
N4-Acetylcytidine triphosphate sodium serves as an efficient substrate in T7 Polymerase-driven in vitro transcription reactions, demonstrating its ability to be successfully incorporated into various templates. This modified nucleotide offers a unique advantage in expanding the scope of RNA synthesis, enabling the production of acetylated RNA molecules with potential applications in research and therapeutic development. Its compatibility with T7 Polymerase highlights its utility in generating tailored RNA constructs, providing researchers with a versatile tool for exploring RNA modifications and their functional implications. |
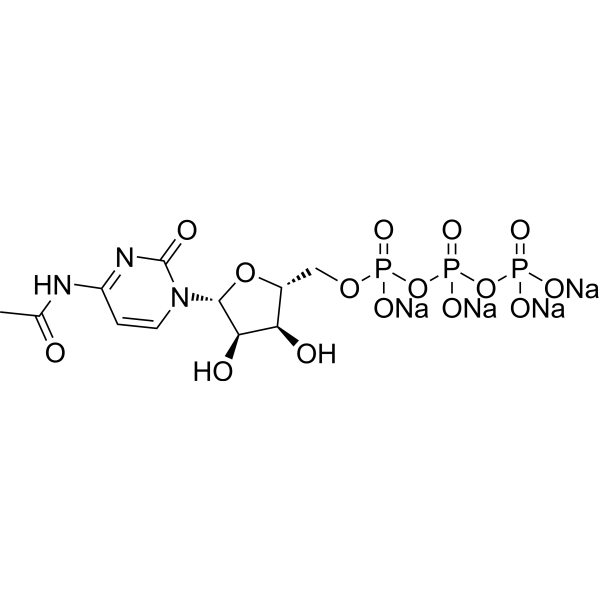
|
| DC67300 |
FBnG
Featured
|
FBnG is a key component of the non-ribosomal peptide synthetase/polyketide synthase (NRPS/PKS) machinery, playing a crucial role in the biosynthesis of fabrubactin (FBN). This versatile molecule can be utilized in the synthesis of AUTAC4, specifically as part of the compound FBnG-(Cys-acetamide)-CH2-PEG3-CH2-CH2-CH2-NH2 (HY-150408). By leveraging its integration into this synthetic pathway, FBnG serves as a valuable building block for the development of AUTAC4, highlighting its potential in advancing research and therapeutic applications related to targeted protein degradation and related biological processes. |
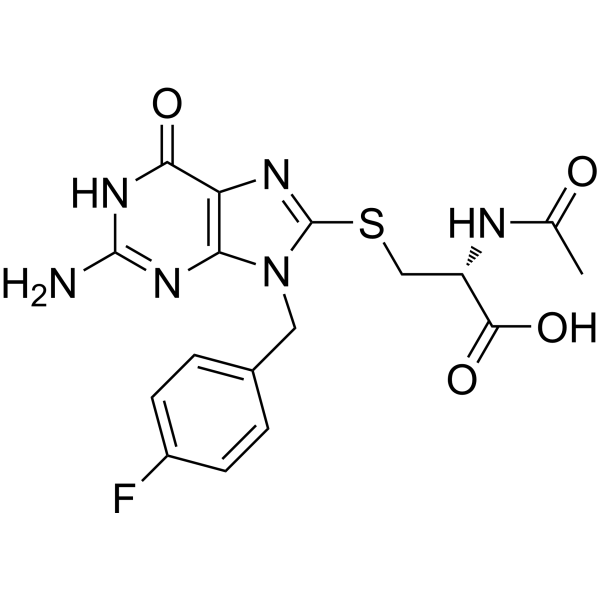
|
| DC67301 |
3-sucCA
Featured
|
3-Succinylated cholic acid (3-sucCA) is a microbially derived bile acid that plays a significant role in gut health and metabolic regulation. As a lumen-restricted metabolite, 3-sucCA has been shown to mitigate the progression of metabolic-associated fatty liver disease (MAFLD) to metabolic-associated steatohepatitis (MASH) in mouse models. Its protective effects are primarily attributed to its ability to reshape the gut microbiota, particularly by enhancing the growth of Akkermansia muciniphila, a beneficial bacterium associated with improved metabolic health. Notably, patients with biopsy-confirmed MAFLD exhibit reduced levels of 3-sucCA, underscoring its potential as a biomarker and therapeutic target for managing metabolic liver diseases. This unique metabolite highlights the intricate interplay between gut microbiota and liver health, offering promising avenues for intervention. |
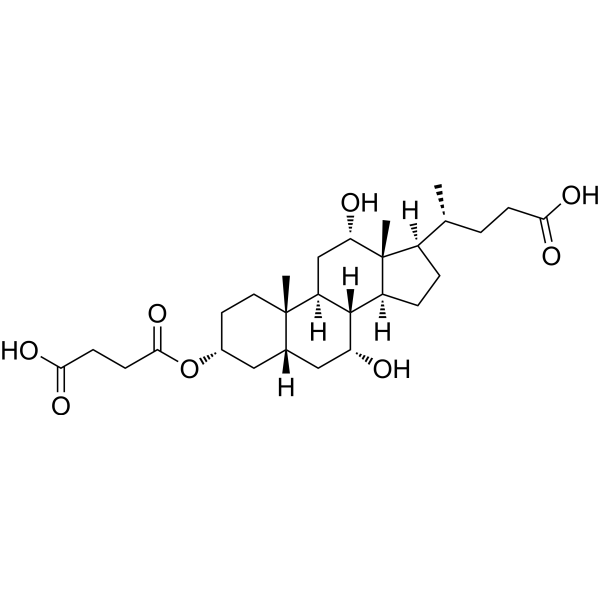
|
| DC67302 |
KAT modulator-1
Featured
|
KAT modulator-1 (Compound 3) is a novel modulator of lysine acetyltransferases (KATs) with a unique mechanism of action. It specifically interacts with the full-length p300 protein but does not engage with its isolated catalytic domain, highlighting its distinctive binding properties. This compound serves as a valuable tool for epigenetics research, enabling the exploration of p300's role in chromatin remodeling, gene regulation, and other epigenetic processes. Its selective interaction with full-length p300 offers insights into the structural and functional complexities of KATs, paving the way for the development of targeted epigenetic therapies. |
|
| DC67303 |
I-152
Featured
|
I-152 is a novel conjugate composed of N-acetyl-cysteine (NAC) and cysteamine (MEA), designed to harness the synergistic effects of these two bioactive compounds. It demonstrates the ability to activate key cellular signaling pathways, including NRF2 and ATF4, which are involved in oxidative stress response and cellular homeostasis. Additionally, I-152 exhibits potent anti-proliferative properties, making it a promising candidate for research in conditions characterized by uncontrolled cell growth, such as cancer. This unique combination of NAC and MEA in I-152 offers a multifaceted approach to modulating cellular pathways and addressing pathological processes. |
|
| DC67304 |
JMV6944
Featured
|
JMV6944 is a potent agonist of the pregnane X receptor (PXR), demonstrating its ability to competitively inhibit the binding of the human PXR ligand-binding domain (LBD) with an IC50 value of 680 nM. This compound effectively induces the expression of CYP3A4 mRNA in freshly isolated primary human hepatocyte cultures, highlighting its role in modulating drug metabolism and detoxification pathways. JMV6944 serves as a valuable tool for investigating PXR-mediated transcriptional regulation and its implications in xenobiotic metabolism, liver function, and therapeutic interventions. Its dual functionality as a PXR agonist and CYP3A4 inducer underscores its potential in both research and drug development. |
|