| Cas No.: | 1715-30-6 |
| Chemical Name: | 2,4,11,13-Tetraazatetradecanediimidamide,N1,N14-bis(2-ethylhexyl)-3,12-diimino-, hydrochloride (1:2) |
| Synonyms: | 2,4,11,13-Tetraazatetradecanediimidamide,N1,N14-bis(2-ethylhexyl)-3,12-diimino-, hydrochloride (1:2);2,4,11,13-Tetraazatetradecanediimidamide,N1,N14-bis(2-ethylhexyl)-3,12-diimino-, hydrochloride...;Alexidine (hydrochloride);Alexidine dihydrochloride;Alexidine dihydrochloride,N,N\'\'-Bis(2-ethylhexyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamidedihydrochloride;Alexidine dihydrochloride,N,N''-Bis(2-ethylhexyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamidedihydrochloride;1,1'-Hexamethylene-bis(5-[2-ethylhexyl]biguanide);alexidine 2HCl;Bisguadine;Bisguanidine;Compound-904;QR-711;Sterwin 904;1,1′-Hexamethylene-bis(5-[2-ethylhexyl]biguanide);WIN-21904;1,1'-Hexamethylenebis[5-(2-ethylhex-1-yl)]biguanide dihydrochloride;N,N''-Bis(2-ethylhexyl)-3,12-diiMino-2,4,11,13-tetraazatetradecanediiMidaMide;N,N''-bis(2-ethylhexyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediamidine dihydrochloride;N,N''-Bis(2-ethylhexyl)-3,12-diimino-2,4,11,13-tetraazatetradecanediimidamidedihydrochloride |
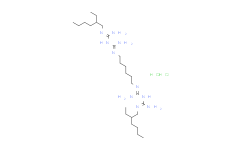
| SMILES: | Cl.Cl.CCCCC(C/N=C(/N/C(=N/CCCCCC/N=C(/N/C(=N/CC(CCCC)CC)/N)\N)/N)\N)CC |
| Formula: | C26H58Cl2N10 |
| M.Wt: | 581.7117228508 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Alexidine dihydrochloride is an anticancer agent that targets a mitochondrial tyrosine phosphatase, PTPMT1, in mammalian cells and causes mitochondrial apoptosis. Alexidine dihydrochloride has antifungal and antibiofilm activity against a diverse range of fungal pathogens[1]. |
| Target: | PTPMT1[1] |
| In Vivo: | Chosen to focus on biofilm formation by C. albicans, since a murine biofilm model has been well established in this fungus and used for testing the effects of established and new antifungal agents. The effect of the drugs on the 24-h-old biofilms growing in the jugular vein catheters of mice is visualized microscopically, which reveals significantly lower density of the biofilms in catheters treated with Alexidine dihydrochloride. In fact, fungal CFU determination reveals that Alexidine dihydrochloride inhibits 67% of fungal biofilm growth and viability, compared to the control untreated biofilms[1]. |
| In Vitro: | Alexidine dihydrochloride displays activity against most Candida spp.; MIC values of ≤1.5 μg/mL are observed for all isolates tested under planktonic conditions, with the exception of Candida parapsilosis and Candida krusei. Interestingly, Alexidine dihydrochloride also displays striking activity against clinically relevant fluconazole-resistant Candida isolates: C. albicans (CA2, CA6, and CA10), C. glabrata (CG2 and CG5), C. parapsilosis (CP5), and C. auris (CAU-09 and CAU-03)[1]. Inhibition of planktonic growth by Alexidine dihydrochloride reveals a complete inhibition of filamentation or proliferation of the imaged fungi. Alexidine dihydrochloride is able to decimate at low concentrations (1.5 to 6 μg/mL) mature biofilms of Candida, Cryptococcus, and Aspergillus spp. that are known to be resistant to almost all classes of antifungal drugs. In fact, at 10-fold-lower concentrations (150 ng/mL) of planktonic MICs, Alexidine dihydrochloride could inhibit lateral yeast formation and biofilm dispersal in C. albicans[1]. Alexidine dihydrochloride results in 50% killing of HUVECs and lung epithelial cells, at concentrations 5- to 10-fold higher than the MIC required to kill planktonically growing fungal pathogens[1]. |
| References: | [1]. Mamouei Z, et al. Alexidine Dihydrochloride Has Broad-Spectrum Activities against Diverse Fungal Pathogens. mSphere. 2018 Oct 31;3(5). pii: e00539-18. |

 To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.
To enhance service speed and avoid tariff delays, we've opened a US warehouse. All US orders ship directly from our US facility.