| Cas No.: | 1290541-46-6 |
| Chemical Name: | RAD51 inhibitor B02 |
| Synonyms: | RAD51 inhibitor B02;(E)-3-benzyl-2-(2-(pyridin-3-yl)vinyl)quinazolin-4(3H)-one;B02;3-(Phenylmethyl)-2-[(1E)-2-(3-pyridinyl)ethenyl]-4(3H)-quinazolinone;BO 2;MLS000709026;3-benzyl-2-[(E)-2-pyridin-3-ylethenyl]quinazolin-4-one;SMR000289793;3-Benzyl-2-[(E)-2-(3-pyridinyl)ethenyl]-4(3H)-quinazolinone;RAD51 Inhibitor B02; BO2;BDBM48804;cid_5738263;HMS2612I15;BCP19688;s8434;STK856883;B- |
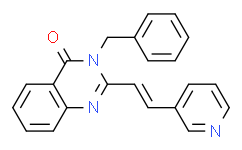
| SMILES: | O=C1C2=C([H])C([H])=C([H])C([H])=C2N=C(/C(/[H])=C(\[H])/C2=C([H])N=C([H])C([H])=C2[H])N1C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H] |
| Formula: | C22H17N3O |
| M.Wt: | 339.3899 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | B02 is a cell-permeable pyridinylvinyl-quinazolinone compound that is shown to specifically inhibit human RAD51 (IC50 = 27.4 µM). Does not affect RecA even at much higher concentration (~250 µM). Directly interacts with RAD51 (Kd = 5.6 µM), and disrupts its binding to DNA and nucleoprotein filament formation. Blocks double-strand break-induced homologous recombination and enhances sensitivity of cells to Cisplatin and Mitomycin C . Diminishes co-aggregate formation between RAD51-ssDNA filament. |
| In Vivo: | B02 significantly enhances the therapeutic effect of cisplatin on tumor cells in vivo. B02 is tolerated by mice at doses up to 50 mg/kg without obvious body weight loss. No inhibition of tumor growth is observed on mice solely treated by B02. Mice treated with 4 mg/kg cisplatin, however, shows a 33% inhibition of tumor growth. Finally, mice treated with 50 mg/kg B02 and 4 mg/kg cisplatin shows a 66% inhibition of tumor growth[2]. |
| In Vitro: | RAD51 Inhibitor B02 specifically inhibits human RAD51 (IC50=27.4 μM), but not its E. coli homologue RecA (IC50>250 μM)[1]. The combination of B02 with cisplatin has the strongest killing effect on the human breast cancer cells MDA-MB-231[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.