| Cas No.: | 688348-33-6 |
| Chemical Name: | Benzene, 1,3-dichloro-5-[(1E)-2-[4-(trifluoromethyl)phenyl]ethenyl]- |
| Synonyms: | Benzene, 1,3-dichloro-5-[(1E)-2-[4-(trifluoromethyl)phenyl]ethenyl]-;1,3-DICHLORO-5-[(1E)-2(4-TRIFLUOROMETHYLPHENYL)ETHENYL]-BENZENE;CAY10465;CAY 10465 |
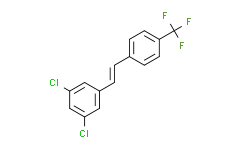
| SMILES: | FC(C1=CC=C(/C=C/C2=CC(Cl)=CC(Cl)=C2)C=C1)(F)F |
| Formula: | C15H9F3Cl2 |
| M.Wt: | 317.13316 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | CAY 10465 is a selective and high-affinity AhR agonist, with a Ki of 0.2 nM, and shows no effect on estrogen receptor (Ki >100000 nM). |
| In Vitro: | CAY 10465 (Compound 4i) is a selective and high-affinity AhR receptor agonist, with a Ki of 0.2 nM, and shows no effect on estrogen receptor (Ki >100000 nM)[1]. CAY 10465 (0.01 nM-100 μM) reduces apolipoprotein A-I (apo A-I) levels in HepG2 cells, and inhibits the synthesis of the protein[2]. |
| References: | [1]. de Medina P, et al. Synthesis and biological properties of new stilbene derivatives of resveratrol as new selective aryl hydrocarbon modulators. J Med Chem. 2005 Jan 13;48(1):287-91. [2]. Naem E, et al. Inhibition of apolipoprotein A-I gene by the aryl hydrocarbon receptor: a potential mechanism for smoking-associated hypoalphalipoproteinemia. Life Sci. 2012 Jul 26;91(1-2):64-9. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.