| Cas No.: | 158081-99-3 |
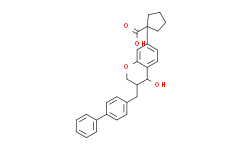
| Chemical Name: | Cyclopentanecarboxylicacid,1-[(3S,4R)-3-([1,1'-biphenyl]-4-ylmethyl)-3,4-dihydro-4-hydroxy-2H-1-benzopyran-7-yl]- |
| Synonyms: | Cyclopentanecarboxylicacid,1-[(3S,4R)-3-([1,1'-biphenyl]-4-ylmethyl)-3,4-dihydro-4-hydroxy-2H-1-benzopyran-7-yl]-;CP 105696;Cyclopentanecarboxylicacid, 1-[3-([1,1'-biphenyl]-4-ylmethyl)-3,4-dihydro-4-hydroxy-2H-1-benzopyran-7-yl]-,(3S-trans)-;Pfizer 105696;(+)-1-(3S,4R)-[3-(4-Phenylbenzyl)-4-hydroxychroman-7-yl]cyclopentane carboxylic acid;1-[(3S,4R)-3-([1,1′-Biphenyl]-4-ylmethyl)-3,4-dihydro-4-hydroxy-2H-1-benzopyran-7-yl]-cyclopentanecarboxylic acid;CP-105696;CP105696 |
| SMILES: | O=C(C1(C2=CC=C3[C@H](O)[C@@H](CC4=CC=C(C5=CC=CC=C5)C=C4)COC3=C2)CCCC1)O |
| Formula: | C28H28O4 |
| M.Wt: | 428.51952 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | CP-105696 is a potent and selective Leukotriene B4 Receptor antagonist, with an IC50 of 8.42 nM. |
| In Vivo: | At a dose of 50 mg/kg/day (28 days), B10.BR (H2k) allografts transplanted into C57Bl/6 (H2b) recipients are significantly protected, as reflected by the mean survival time versus control grafts (27±20 days [n=10] vs. 12±6 days [n=14]; P=0.0146). Using an induction protocol (day -1 to day 3), CP-105696 at 100 mg/kg/day significantly prolongs allograft survival (33±23 days [n=9]; P=0.0026), but CP-105696 at 10 mg/kg/day does not (18±16 days [n=8]; P=0.1433). Syngeneic grafts survive indefinitely (n=11). Immunohistological evaluation of allografts at rejection reveals a mononuclear cell infiltrate composed primarily of CD3+ and CD11b+ (Mac-1+) cells, which are infrequent in syngeneic grafts. Allografts from mice treated with CP-105696 at 50 or 100 mg/kg/day demonstrat a selective reduction in β2-integrin (Mac-1) expression on monocytes/macrophages, as demonstrated by CD11b staining density compared with allograft controls[2]. |
| In Vitro: | CP-105696 is a structurally novel, selective and potent LTB4 receptor antagonist. In vitro, CP-105696 inhibits [3H]LTB4 (0.3 nM) binding to high-affinity LTB4 receptors on human neutrophils with an lC50 value of 8.42±0.26 nM. Scatchard analyses of [3H]LTB4 binding to these high-affinity receptors indicate that CP-105696 acts as a noncompetitive antagonist. CP-105696 inhibits human neutrophil chemotaxis mediated by LTB4 (5 nM) in a noncompetitive manner with an IC50 value of 5.0±2.0 nM. Scatchard analyses of [3H]LTB4 binding to low-affinity receptors on neutrophils indicate that CP-105696 acts as a competitive antagonist at this receptor, and inhibition of LTB4-mediated CD11b upregulation on human neutrophils is competitively inhibited by CP-105696 (pA2=8.03±0.19). CP-105696 at 10 μM does not inhibit either human neutrophil chemotaxis or CD11b upregulation mediated through alternate (i.e., C5a, lL-8, PAF) G-protein coupled chemotactic factor receptors. In isolated human monocytes, LTB4 (5 nM)-mediated Ca2+ mobilization is inhibited by CP-105696 with an lC50 value of 940±70 nM[1]. |
| Animal Administration: | Mice[2] Allogeneic donor hearts are harvested after intravenous heparinization of donor B10.BR mice (H2k) and are preserved via retrograde perfusion with cold cardioplegia solution into the left ventricle. Recipient C57Bl/6 mice (H2b) are prepared by ligating the lumbar vessels and isolating the abdominal aorta and vena cava; donor hearts are sutured in place by microvascular anastomoses of the donor aorta and pulmonary artery to the recipient aorta and inferior vena cava, respectively. CP-105696 is evaluated in a 28-day treatment protocol (50 mg/kg/day), a high-dose (100 mg/kg/day) induction protocol (day -1 to day 3), and a low-dose (10 mg/kg/day) induction protocol (day -1 to day 3). In all study groups, drug is administered orally in a 0.5% methylcellulose vehicle. In parallel studies, treatment of C57Bl/6 (H2b) recipients bearing B10.BR (H2k) cardiac allografts given FK506 (2 mg/kg/day for 28 days), our standard control immunosuppressant, significantly prolongs allograft survival (mean survival time [MST], 40±18 days [n=9]; P=0.0002)[2]. |
| References: | [1]. Showell HJ, et al. The in vitro and in vivo pharmacologic activity of the potent and selective leukotriene B4 receptor antagonist CP-105696. J Pharmacol Exp Ther. 1995 Apr;273(1):176-84. [2]. Weringer EJ, et al. Antagonizing leukotriene B4 receptors delays cardiac allograft rejection in mice. Transplantation. 1999 Mar 27;67(6):808-15. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.