| Cas No.: | 864070-44-0 |
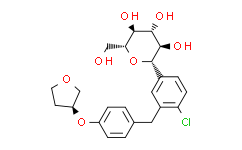
| Chemical Name: | Empagliflozin |
| Synonyms: | Empagliflozin;Empagliflozin (BI 10773);(2S,3R,4R,5S,6R)-2-[4-chloro-3-[[4-[(3S)-oxolan-3-yl]oxyphenyl]methyl]phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol;BI 10773;BI-10773;JARDIANCE;BI10773;HDC1R2M35U;1-chloro-4-(glucopyranos-1-yl)-2-(4-(tetrahydrofuran-3-yloxy)benzyl)benzene;(1S)-1,5-anhydro-1-(4-chloro-3-{4-[(3S)-tetrahydrofuran-3-yloxy]benzyl}phenyl)-D-glucitol;(2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-(((S)-tetrahydrofuran-3-yl)oxy)benzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol;(1S)-1,5-anhydro-1-C-{4-chloro-3-((4-{((3S)-oxolan-3-yl)oxy}phenyl)methyl)pheny |
| SMILES: | ClC1C([H])=C([H])C(=C([H])C=1C([H])([H])C1C([H])=C([H])C(=C([H])C=1[H])O[C@]1([H])C([H])([H])OC([H])([H])C1([H])[H])[C@@]1([H])[C@@]([H])([C@]([H])([C@@]([H])([C@@]([H])(C([H])([H])O[H])O1)O[H])O[H])O[H] |
| Formula: | C23H27ClO7 |
| M.Wt: | 450.9093 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | Empagliflozin is a selective sodium glucose cotransporter-2 (SGLT-2) inhibitor with an IC50 of 3.1 nM for human SGLT-2.. |
| In Vivo: | Glucose intolerance is significantly improved after 8 days of Empagliflozin treatment at either dose (3mg/kg Empagliflozin 3058±180 vs 10mg/kg Empagliflozin 3090±219). Therefore, acute treatment with Empagliflozin has a beneficial effect on hyperglycemia and glucose intolerance. Since there are no significant differences in blood glucose homeostasis with the two different doses of Empagliflozin, and random blood glucose levels of T1DM mice are significantly improved by 3mg/kg of Empagliflozin, the effect of the lower dose of Empagliflozin (3mg/kg) is investigated on preserving β-cell mass and function[2]. |
| In Vitro: | Empagliflozin is a potent and competitive SGLT-2 inhibitor with an excellent selectivity profile and the highest selectivity window of the tested SGLT-2 inhibitors over hSGLT-1. Empagliflozin inhibits the uptake of [14C]-alpha-methyl glucopyranoside (AMG) via hSGLT-2 in a dose-dependent manner with an IC50 of 3.1 nM, but is less potent for other SGLTs (IC50 range: 1100-11000 nM). [3H]-Empagliflozin displays a high affinity for SGLT-2 with a mean Kd of 57±37 nM in the absence of glucose in kinetic binding experiments[1]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.