| Cas No.: | 71827-03-7 |
| Chemical Name: | Ivermectin B1a |
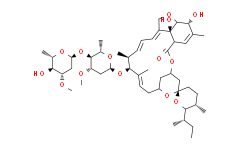
| SMILES: | C/C([C@H]([C@H](/C=C/C=C1CO[C@]([H])([C@@H](C(C)=C[C@]2(C3=O)[H])O)[C@@]\12O)C)O[C@]4(C[C@@H]([C@H]([C@@H](O4)C)O[C@@]5(O[C@H]([C@@H]([C@H](C5)OC)O)C)[H])OC)[H])=C\C[C@@]6(C[C@@]([H])(C[C@@]7(O6)CC[C@@H]([C@]([H])(O7)[C@@H](C)CC)C)O3)[H] |
| Formula: | C48H74O14 |
| M.Wt: | 875.09 |
| Purity: | >98% |
| Sotrage: | Powder-20°C3 yearsIn solvent-80°C6 months-20°C1 month |
| Description: | Ivermectin B1a, a derivative of Avermectin B1a, is a main component of Ivermectin. Ivermectin (MK-933) is a broad-spectrum anti-parasite agent. Ivermectin is a candidate therapeutic against SARS-CoV-2/COVID-19. |
| In Vitro: | Ivermectin belongs to the macrocyclic lactone class of avermectins and consists of a mixture of two homologous compounds, ivermectin B1a (not less than 80%) and ivermectin B1b (not more than 20%). Ivermectin B1a, the major component of Ivermectin, is inactive at a concentration of 0.3 μg/ml, whereas in the case of the minor component, ivermectin B1b, the same concentration produces 100% mortality of snails[1]. |
| References: | [1]. Naftale Kat, et al. Ivermectin Efficacy Against Biomphalaria, Intermediate Host Snail Vectors of Schistosomiasis. J Antibiot (Tokyo). 2017 May;70(5):680-684. [2]. Khan Sharun, et al. Ivermectin, a New Candidate Therapeutic Against SARS-CoV-2/COVID-19. Ann Clin Microbiol Antimicrob. 2020 May 30;19(1):23. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.