| Cas No.: | 198419-91-9 |
| Chemical Name: | LY 367385 |
| Synonyms: | Benzeneacetic acid, a-amino-4-carboxy-2-methyl-, (aS)-;LY 367385;AC1NSKBK;CHEBI:252690;CHEMBL94631;LY-367385;LY-367385 hydrochloride;PDSP1_001314;PDSP2_001298;SureCN661700;Tocris-1237;SGIKDIUCJAUSRD-QMMMGPOBSA-N;(S)-4-[aMino(carboxy)Methyl]-3-Methylbenzoic acid;(S)-(+)-α-Amino-4-carboxy-2-methylbenzeneaceticacid;(S)-(+)-a-amino-4-carboxy-2-methylbenzeneacetic acid;(R)-(+)-alpha-Amino-4-carboxy-2-methylbenzeneacetic acid;(S)-(+)-ALPHA-AMINO-4-CARBOXY-2-METHYLBENZENEACETIC ACID;LY367385;(S)-(+)-?-Amino-4-carboxy-2-methylbenzeneacetic acid;D8UW47H17B;4-[(S)-amino(carboxy)methyl]-3-methylbenzoic acid;(s)-(+)-alpha-amino-4-carboxy-2-methylbenzeneacetic acid;CS-0028681;SR-01000597418-1;2-methyl-4-carboxyphenylglycine;D04JQR;198419-91-9;SR-01000597418;NCGC00025067-01;NCGC00025067-02;(s)-2-methyl-4-carboxyphenylglycine;(S)-(+)-alpha-Amino-4-carboxy-2-methylbenzeneaceticacid;BENZENEACETIC ACID, .ALPHA.-AMINO-4-CARBOXY-2-METHYL-, (.ALPHA.S)-;Benzeneacetic acid, alpha-amino-4-carboxy-2-methyl-, (S)-;BDBM50084140;(S)-2-(4-Carboxy-2-methylphenyl)glycine;(S)-4-(amino(carboxy)methyl)-3-methylbenzoic acid;GTPL1379;Metabotropic mGluR1 agonists, Lilly;UNII-D8UW47H17B;YHA41991;BENZENEACETIC ACID, .ALPHA.-AMINO-4-CARBOXY-2-METHYL-, (S)-;4-((S)-AMINO(CARBOXY)METHYL)-3-METHYLBENZOIC ACID;HB0398;SCHEMBL661700;HY-107515;DTXSID2042563;(+)-2-methyl-4-carboxyphenylglycine;4-[(1S)-1-amino-2-hydroxy-2-oxoethyl]-3-methylbenzoic acid;J-012816;Benzeneacetic acid, alpha-amino-4-carboxy-2-methyl-, (alphaS)-;MS-23147;Q27083034;Benzeneacetic acid,a-amino-4-carboxy-2-methyl-,(as)-;4-((S)-Amino-carboxy-methyl)-3-methyl-benzoic acid;LY-367385 hydrochloride, >=98% (HPLC);AKOS015923884 |
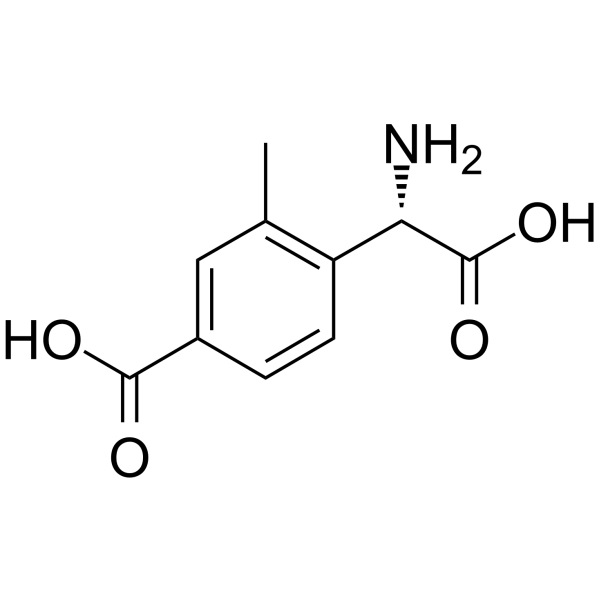
| SMILES: | OC([C@H](C1C=CC(C(=O)O)=CC=1C)N)=O |
| Formula: | C10H11NO4 |
| M.Wt: | 209.198642969131 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | LY367385 is a highly potent and selective mGluR1a antagonist. LY367385 has an IC50 of 8.8 μM for inhibits of quisqualate-induced phosphoinositide (PI) hydrolysis, compared with > 100 μM for mGlu5a. LY367385 has neuroprotective, anticonvulsant and antiepileptic effects[1][2]. |
| Target: | mGluR1a:8.8 μM (IC50) |
| In Vivo: | LY367385 has been administered intracerebroventricularly (i.c.v.) to DBA/2 mice and lethargic mice (lh/lh), and focally into the inferior colliculus of genetically epilepsy prone rats (GEPR). In DBA/2 mice, LY367385 produces a rapid, transient suppression of sound-induced clonic seizures ED50 = 12 nM, i.c.v., 5 min). In lethargic mice, LY367385 significantly reduces the incidence of spontaneous spike and wave discharges on the electroencephalogram, from 30 to >150 min after the administration of LY367385, 250 nM, i.c.v[3]. In genetically epilepsy prone rats, LY367385 reduces sound-induced clonic seizures. LY367385, 160 nM bilaterally, fully suppresses clonic seizures after 2-4 h[3]. |
| In Vitro: | LY367385 combined with N-methyl-D-aspartate (NMDA) during the toxic pulse attenuates neuronal degeneration in a concentration-dependent fashion, with a maximal reduction of NMDA toxicity ranging from 40 to 60%. LY367385 shows greater efficacy than LY367366 and neither of these compounds influenced neuronal viability per se. LY367385 shows potent neuroprotective effects, with causing a 50% reduction in (S)-3,5-Dihydroxyphenylglycine (DHPG) potentiation at a concentration of 10 nM. Under experimental conditions at higher concentrations of antagonist, LY367385 a completely antagonized the amplification of NMDA toxicity by DHPG[2]. |
| References: | [1]. Clark et al. (+)-2-Methyl-4-carboxyphenylglycine (LY 367385) selectively antagonises metabotropic glutamate mGluR1 receptors. Bioorg.Med.Chem.Lett. November 1997, 7 (21): 2777-2780. [2]. Bruno V, et al. Neuroprotective activity of the potent and selective mGlu1a metabotropic glutamate receptor antagonist, (+)-2-methyl-4 carboxyphenylglycine (LY367385): comparison with LY357366, a broader spectrum antagonist with equal affinity for mGlu1a and mGlu5 receptors. Neuropharmacology. 1999 Feb;38(2):199-207. [3]. Chapman AG, et al. Anticonvulsant actions of LY 367385 ((+)-2-methyl-4-carboxyphenylglycine) and AIDA ((RS)-1-aminoindan-1,5-dicarboxylic acid). Eur J Pharmacol. 1999 Feb 26;368(1):17-24. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.