| Cas No.: | 1262417-51-5 |
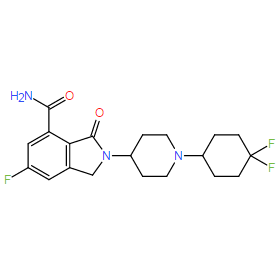
| Chemical Name: | 2-[1-(4,4-difluorocyclohexyl)piperidin-4-yl]-6-fluoro-3-oxo-2,3-dihydro-1H-isoindole-4-carboxamide |
| Synonyms: | NMS-P118; NMS-P 118; NMS P118 |
| SMILES: | O=C(C1=CC(F)=CC(CN2C3CCN(C4CCC(F)(F)CC4)CC3)=C1C2=O)N |
| Formula: | C20H24F3N3O2 |
| M.Wt: | 395.43 |
| Purity: | >98% |
| Sotrage: | 4°C for 1 year, -20°C for more than 2 years |
| Description: | NMS-P118 is a potent, orally available, and highly selective PARP-1 Inhibitor for cancer therapy. |
| Target: | PARP-1:9 nM (Kd) PARP-2:1390 nM (Kd) |
| In Vivo: | NMS-P118 is a potent (KD=0.009 μM) PARP-1 inhibitor, showing 150-fold selectivity over PARP-2 (KD=1.39 μM). NMS-P118 possesses excellent pharmacokinetic profile and nearly complete oral bioavailability both in mice and rats. It proved to be highly efficacious in vivo both as single agent in MDA-MB-436 human breast cancer tumors and in combination with temozolomide in CAPAN-1 human pancreatic tumors growing as xenografts in the mouse. The compound is well tolerated at highly efficacious doses and is endowed with an excellent ADME profile[1]. |
| In Vitro: | NMS-P118 is found to be less myelotoxic in vitro than olaparib (now marketed as Lynparza), a dual PARP-1/-2 inhibitor. NMS-P118 proves to be metabolically stable, it modestly inhibites two cytochrome P450 family members (CYP-2B6 IC50: 8.15 μM; CYP-2D6 IC50: 9.51 μM) out of eight isoforms tested. Its ability in hampering the proliferation of bone marrow cells is from 5 to > 60 times lower then olaparib according to the species[1]. |
| Kinase Assay: | NMS-P118 is profiled on 56 different kinases (ABL, ACK1, AKT1, ALK, AUR1, AUR2, BRK, BUB1, CDC7/DBF4, CDK2/CYCA, CHK1, CK2, EEF2K, EGFR1, ERK2, EphA2, FAK, FGFR1, FLT3, GSK3beta, Haspin, IGFR1, IKK2, IR, JAK1, JAK2, JAK3, KIT, LCK, LYN, MAPKAPK2, MELK, MET, MNK2, MPS1, MST4, NEK6, NIM1, P38alpha, PAK4, POLYDATINGFRb, POLYDATINK1, PERK, PIM1, PIM2, PKAalpha, PKCbeta, PLK1, RET, SULU1, Syk, TLK2, TRKA, TYK2, VEGFR2, ZAP70). The IC50 values are found to be >10 μM for all enzymes tested[1]. |
| Cell Assay: | NMS-P118 is dissolved in DMSO and diluted with appropriate medium before use. Cellular activity of PARP-1 inhibitors is assessed by measuring the inhibition of the hydrogen peroxide induced PAR formation in HeLa cells (ECACC). Cellular PAR levels are measured by immunocytochemistry, and quantified using an ArrayScan vTi instrument[1]. |
| Animal Administration: | The pharmacokinetic profile and the oral bioavailability of the compounds have been investigated in rat in ad hoc pharmacokinetic studies. NMS-P118 is formulated for intravenous bolus administration in 20% DMSO + 40% PEG 400 in 5% dextrose. Oral administration is performed using a NMS-P118 suspension in 0.5% methylcellulose. A single administration at the dose of 10 mg/kg for each route and a single oral administration at the dose of 100 mg/kg are given. Three male animals for each study are used[1]. |
| References: | [1]. Papeo G, et al. Discovery of 2-[1-(4,4-Difluorocyclohexyl)piperidin-4-yl]-6-fluoro-3-oxo-2,3-dihydro-1H-isoindole-4-carboxamide (NMS-P118): A Potent, Orally Available, and Highly Selective PARP-1 Inhibitor for Cancer Therapy. J Med Chem. 2015 Sep 10;58(17):6875-98. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.