| Cas No.: | 701213-36-7 |
| Chemical Name: | BMS-626529 |
| Synonyms: | 1-(4-benzoylpiperazin-1-yl)-2-[4-methoxy-7-(3-methyl-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]ethane-1,2-dione;1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-piperazine;BMS-626529;SureCN760768;HY-15440;CS-0938;Piperazine, 1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl]-;BMS626529;AGN-PC-006NYV;1-(4-benzoyl-piperazin-1-yl)-2-[4-methoxy-7-(3-methyl-[1,2,4]triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-ethane-1,2-dione;Piperazine,1-benzoyl-4-[2-[4-methoxy-7-(3-methyl-1H-1,2,4-triazol-1-yl)-1H-pyrrolo[2,3-c]pyridin-3-yl]-1,2-dioxoethyl];Temsavir |
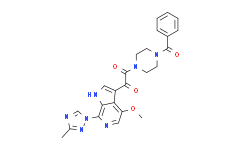
| SMILES: | O=C(N1CCN(C(C2=CC=CC=C2)=O)CC1)C(C3=CNC4=C3C(OC)=CN=C4N5C=NC(C)=N5)=O |
| Formula: | C24H23N7O4 |
| M.Wt: | 473.48392 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | BMS-626529 is a novel attachment inhibitor that targets HIV-1 gp120 and prevents its binding to CD4+ T cells. |
| In Vitro: | BMS-626529 has half-maximal effective concentration (EC50) values of <10 nM against the vast majority of viral isolates. BMS-626529 exhibits an average EC50 against LAI virus of 0.7±0.4 nM. BMS-626529 exhibits an EC50 of 0.01 nM against the most susceptible virus and an EC50 of >2,000 nM against the least susceptible virus. The cytotoxicity profile of BMS-626529 is examined in several cell types from different human tissues. CC50 values of >200 μM are observed in MT-2 (T lymphocytes), HEK293 (kidney), HEp-2 (larynx), HepG2 (liver), HeLa (cervix), HCT116 (colorectal), MCF-7 (breast), SK-N-MC (neuroepithelium), HOS (bone), H292 (lung), and MDBK (bovine kidney) cells measured after 3 or 6 days in culture. CC50 values of 105 and 192 μM are obtained in the T-cell line PM1 and in PBMCs, respectively, following 6 days in culture. These results show that BMS-626529 exhibits low cytotoxicity in cell culture[1]. BMS-626529 exhibits a broad spectrum of antiviral activity against a panel of clinical isolates, with a 50% inhibitory concentration (IC50) ranging from subnanomolar levels to >0.1 µM[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.