| Cas No.: | 749886-87-1 |
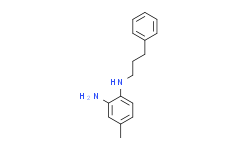
| Chemical Name: | 4-Methyl-N1-(3-phenylpropyl)benzene-1,2-diamine |
| Synonyms: | 4-METHYL-N1-(3-PHENYLPROPYL)BENZENE-1,2-DIAMINE;JSH-23;LogP;4-Methyl-N1-(3-phenylpropyl)-1,2-phenylenediamine;4-methyl-1-N-(3-phenylpropyl)benzene-1,2-diamine;B Activation Inhibitor II, JSH-23;JSH 23;NF-κ4-methyl-N1-(3-phenylpropyl)-1,2-benzenediamine;AK175962;JSH23;4-Methyl-N~1~-(3-phenylpropyl)benzene-1,2-diamine;AOB2674;NF-kappaB activation inhibitor II;HMS3653C16;BCP09944;FD5025;IN1201;s7351;KS-0 |
| SMILES: | N([H])(C1C([H])=C([H])C(C([H])([H])[H])=C([H])C=1N([H])[H])C([H])([H])C([H])([H])C([H])([H])C1C([H])=C([H])C([H])=C([H])C=1[H] |
| Formula: | C16H20N2 |
| M.Wt: | 240.3434 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | JSH-23 is an NF-κB inhibitor which inhibits NF-κB transcriptional activity with an IC50 of 7.1 μM. |
| In Vivo: | JSH-23 (1 and 3 mg/kg, p.o.) significantly reverses the nerve conduction and nerve blood flow deficits seen in diabetic animals and decreases the nerve lipid peroxidation, partially replenishes the depleted levels of GSH in nerve of diabetic rats[4]. |
| In Vitro: | JSH-23 inhibits lipopolysaccharide (LPS)-induced chromatin condensation in a dose-dependent manner, corresponding to 44±4% inhibition at 3 μM, 63±5% at 10 μM and 93±3% at 30 μM[1]. JSH-23 (5, 10, and 15 μM) significantly reduces mean neuronal migration in LPS-activated cells[2]. Co-treatment of A2780 cells with JSH-23 and clinically ineffective transplatin at their IC50 concentrations (130 μM transplatin and 20 μM JSH-23) for 72 h also causes a more pronounced decrease in cell viability compared to the effects of transplatin or JSH-23 alone[3]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.