| Cas No.: | 155877-83-1 |
| Chemical Name: | Benzoic acid,4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)- |
| Synonyms: | Benzoic acid,4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)-;LE 135;4-(7,8,9,10-TETRAHYDRO-5,7,7,10,10-PENTAMETHYL-5H-BENZO[E]NAPHTHO[2,3-B][1,4]DIAZEPIN-13-YL)BENZOIC ACID;4-(5,7,7,10,10-pentamethyl-8,9-dihydronaphtho[2,3-b][1,4]benzodiazepin-13-yl)benzoic acid;HMS3268H07;4-(7,8,9,10-Tetrahydro-5,7,7,10,10-pentamethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)-benzoic acid;LE-135;4-(5,7,7,10,10-Pentamethyl-7,8,9,10-tetrahydro-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)benzoic acid;4-(7,8,9,10-Tetrahydro-5,7,7,10,10-pentamethyl-(5H)-benzo[e]naphtho[2.3-b][1.4]diazepine-13-yl)benzoicacid;Benzoic acid, 4-(7,8,9,10-tetrahydro-5,7,7,10,10-pentaMethyl-5H-benzo[e]naphtho[2,3-b][1,4]diazepin-13-yl)- |
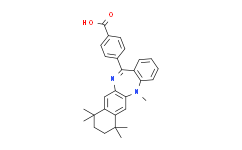
| SMILES: | CN1C2C=CC=CC=2C(C2C=CC(C(O)=O)=CC=2)=NC2C=C3C(=CC1=2)C(C)(C)CCC3(C)C |
| Formula: | C29H30N2O2 |
| M.Wt: | 438.560707569122 |
| Purity: | >98% |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | LE135 is a potent RAR antagonist that binds selectively to RARα (Ki of 1.4 μM) and RARβ (Ki of 220 nM), and has a higher affinity to RARβ. LE135 is highly selective over RARγ, RXRα, RXRβ and RXRγ. LE135 is also a potent TRPV1 and TRPA1 receptors activator with EC50s of 2.5 μM and 20 μM, respectively[1][2]. |
| Target: | RARβ:220 nM (Ki) RARα:1400 nM (Ki) TRPV1:2.5 μM (EC50) TRPA1:20 μM (EC50) |
| In Vivo: | LE135 provokes nociceptive responses and elicited thermal hyperalgesia mainly through TRPV1 channels, but required both TRPA1 and TRPV1 channels for producing mechanical allodynia. Intraplantar injection of LE135 (30 nmol/10 μL) induces mechanical hypersensitivity in wild-type and Trpa1−/− mice[2]. |
| In Vitro: | LE135 inhibits Am80-induced differentiation of human promyelocytic leukemia cells HL-60 with an IC50 of 150 nM[1]. LE135 inhibits retinoic acid (RA)-induced transcriptional activation of RARβ, but not RARα, RARγ or retinoid X receptor α (RXRα), on a variety of RA response elements. LE135 strongly represses 12-O-tetradecanoylphorbol-13-acetate-induced AP-1 activity in the presence of RARβ and RXRα[3]. |
| References: | [1]. H Umemiya, et al. Regulation of retinoidal actions by diazepinylbenzoic acids. Retinoid synergists which activate the RXR-RAR heterodimers. J Med Chem. 1997 Dec 19;40(26):4222-34. [2]. Shijin Yin, et al. LE135, a retinoid acid receptor antagonist, produces pain through direct activation of TRP channels. Br J Pharmacol. 2014 Mar;171(6):1510-20. [3]. Y Li, et al. Identification of a novel class of retinoic acid receptor beta-selective retinoid antagonists and their inhibitory effects on AP-1 activity and retinoic acid-induced apoptosis in human breast cancer cells. J Biol Chem. 1999 May 28;274(22):15360-6. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.