| Cas No.: | 914471-09-3 |
| Chemical Name: | INCB14943 |
| Synonyms: | INCB024360;INCB024360 analogue;4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadiazole-3 -carboximidamide;IDO-IN2;IDO-IN-2;INCB-24360;IDO5L;4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboximidamide;INCB 024360-analog;4-Amino-N-(3-chloro-4-fluorophenyl)-N'-hydroxy-1,2,5-oxadiazole-3-carboxamidine;2HR315841W;IDO inhibitor 5l;INCB14943;AOB6408;HGXSLPIXNPASGZ-UHFFFAOYSA-N;HMS3653H15;BDBM347045;BCP08453;US10202388, Ref. B;s7587;ABP00 |
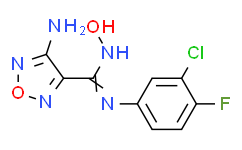
| SMILES: | ClC1=C(C([H])=C([H])C(=C1[H])/N=C(/C1C(N([H])[H])=NON=1)\N([H])O[H])F |
| Formula: | C9H7ClFN5O2 |
| M.Wt: | 271.63 |
| Sotrage: | 2 years -20°C Powder, 2 weeks 4°C in DMSO, 6 months -80°C in DMSO |
| Description: | IDO5L is a potent indoleamine 2,3-dioxygenase (IDO) inhibitor with an IC50 of 67 nM. |
| In Vivo: | Testing of IDO5L in mice demonstrates pharmacodynamic inhibition of IDO, as measured by decreased kynurenine levels (>50%) in plasma and dose dependent efficacy in mice bearing GM-CSF-secreting B16 melanoma tumors. Initial oral pharmacokinetic studies show that IDO5L is rapidly cleared (t1/2<0.5 h) and that oral administration will not be a suitable dosing method for in vivo studies. The measured plasma exposure (2.5 μM) of IDO5L during this period exceeded our calculated mouse protein binding adjusted B16 cellular IC50 (PBadjIC50=1.0 μM, murine cellular B16 IC50=46 nM). Notably, kynurenine levels increase back to baseline after 4 h as IDO5L exposure levels decreased below the mouse PBadjIC50 from 1.0 to 0.1 μM[1]. |
| In Vitro: | IDO5L (Compound 5l) is a potent (HeLa IC50=19 nM) inhibitor of IDO[1]. IDO5L is one of the highest potent inhibitors of the IDO1 (IC50=19 nM, in HeLa cell assay)[2]. |

 DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.
DC Chemicals' products qualify for U.S. tariff exemptions. We guarantee no price increases due to customs duties and maintain stable supply, continuing to deliver reliable research solutions to our American clients.